Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
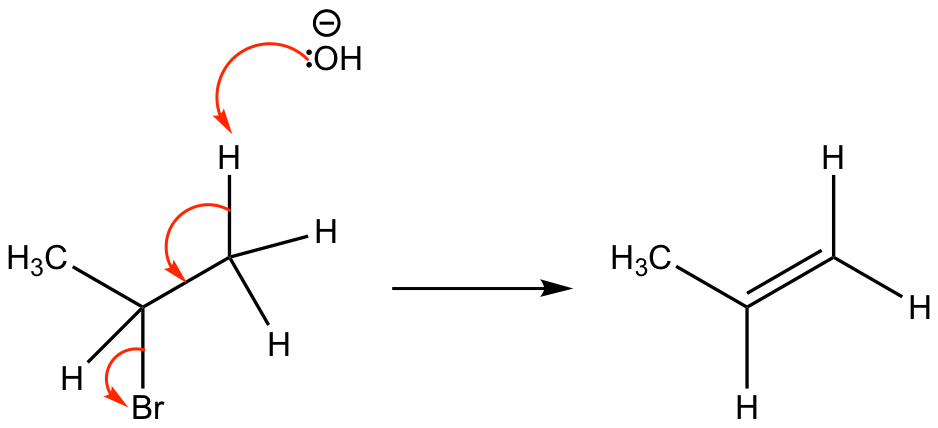
This is an elimination reaction, in which the hydroxide group (acting as a base) will deprotonate the alkane, leading to the formation of a new carbon – carbon double bond. The bromide leaving group then leaves leaving an alkene, water and a bromide ion.
The equation is:
![]()