NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
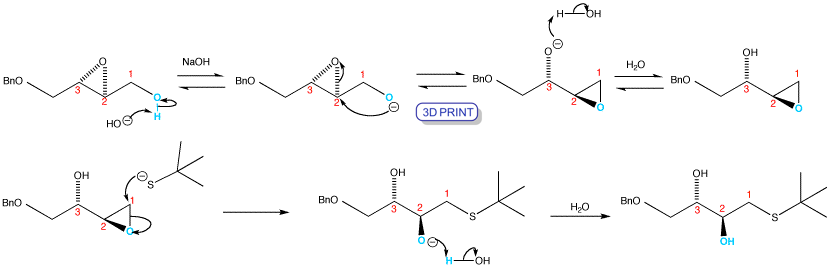
Click the structures and reaction arrows to view the 3D models and animations respectively
t-BuS– is a bulky nucleophile, so direct attack on the secondary epoxide is slow. Under basic conditions the neighbouring alkoxide group attacks intramolecularly to make a new, rearranged epoxy alcohol. The new reactive primary electrophilic site undergoes an SN2 reaction with the t-BuS– under the conditions of the rearrangement. Notice how the blue OH, which started on carbon 1, has ended up on carbon 2.