Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
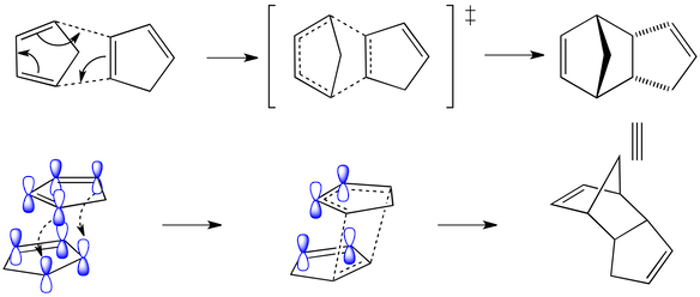
The endo product must have a lower energy transition state (not final structure!) than the exo product. This is why it forms more quickly. By looking at the HOMO and LUMO (frontier orbitals) of the reacting components we can see why.
Orbitals at the back of the diene have the correct symmetry for bonding with aligned counterparts in the dienophile (arbitrary choice in this dimerisation example).
No bonds form, but this favourable through space interaction, explains why the endo product forms fastest in the Diels Alder reaction.
Richard Windsor – Undergraduate Final Year Project 2008
I. Fernández and F. M. Bickelhaupt, Chem. – An Asian J., 2016, 11, 3297–3304.