Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
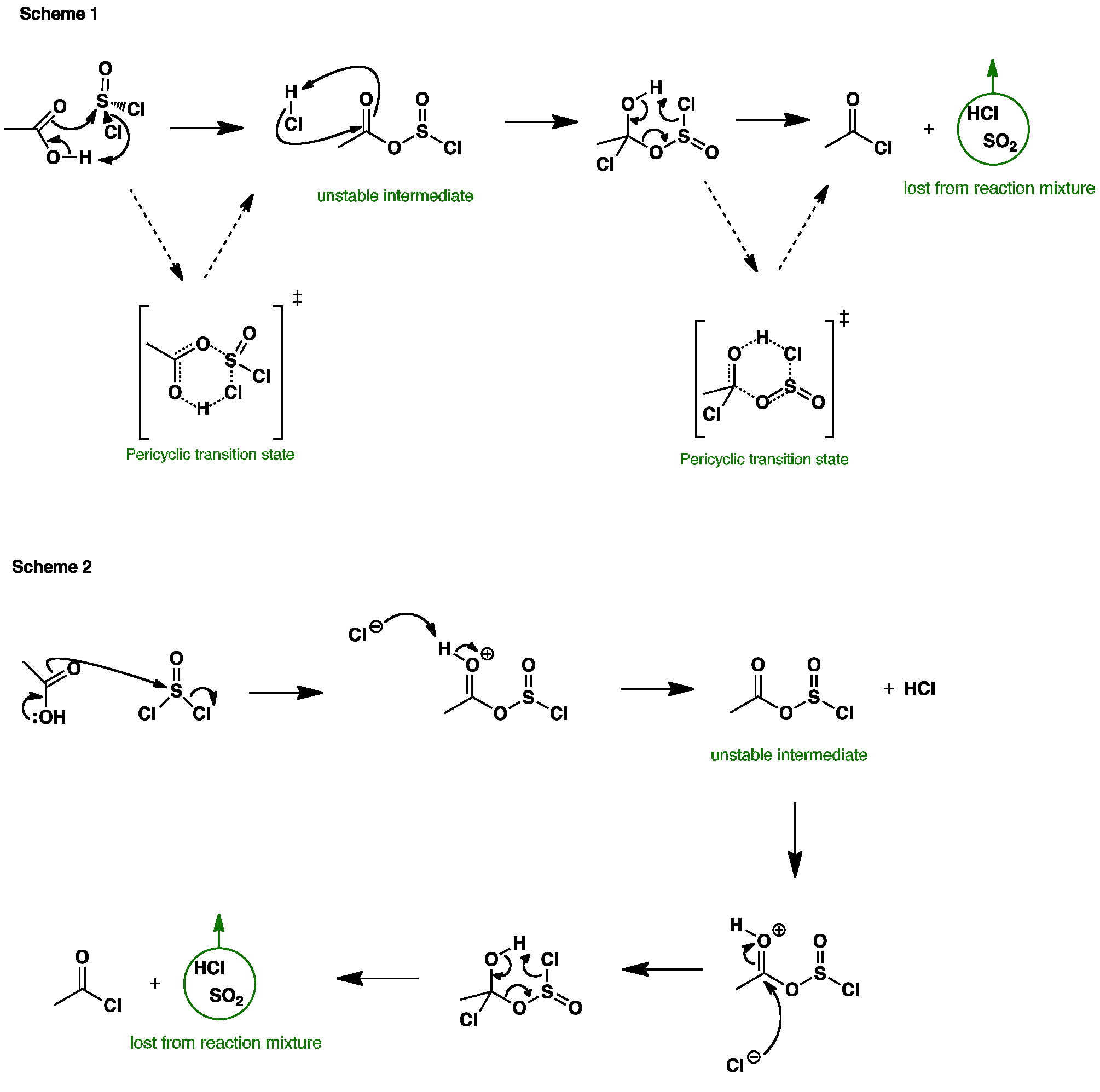
Acid chlorides can be prepared by reacting a carboxylic acid with thionyl chloride SOCl2. Scheme 1, and the animation, shows the pericyclic reaction mechanism for the reaction, whereas scheme 2 shows the ionic reaction mechanism.
The electrophilic sulfur atom is attacked by the carboxylic acid to give an unstable, and highly electrophilic intermediate. This then reacts with the HCl just produced to give a tetrahedral intermediate which then collapses to the acyl chloride, sulfur dioxide and hydrogen chloride. This step is irreversible because SO2 and HCl are gasses that are lost from the reaction mixture.
C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 10827–10852.
M. Green and D. M. Thorp, J. Chem. Soc. B Phys. Org., 1967, 0, 1067.