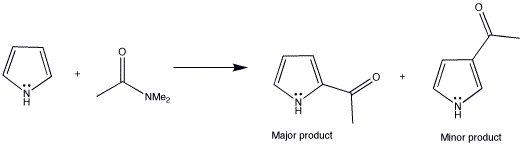
The Vilsmeier reaction is an alternative to Friedel-Crafts acylation and avoids the use of strong Lewis acids such as AlCl3.
This method is particularly useful for formylation because it works well with Me2NCHO (DMF) instead of dimethylacetamide to add a formyl (CHO) group rather than a methyl ketone.
The reaction can be broken down into three stages: formation of an iminium cation, nucleophilic substitution and finally hydrolysis of an iminium salt.
CLICK the stages (for the major product) below to view each 3D animation respectively.
1. Formation of an iminium cation
3. Hydrolysis of an iminium salt
J. Muzart, Tetrahedron, 2009, 65, 8313–8323.