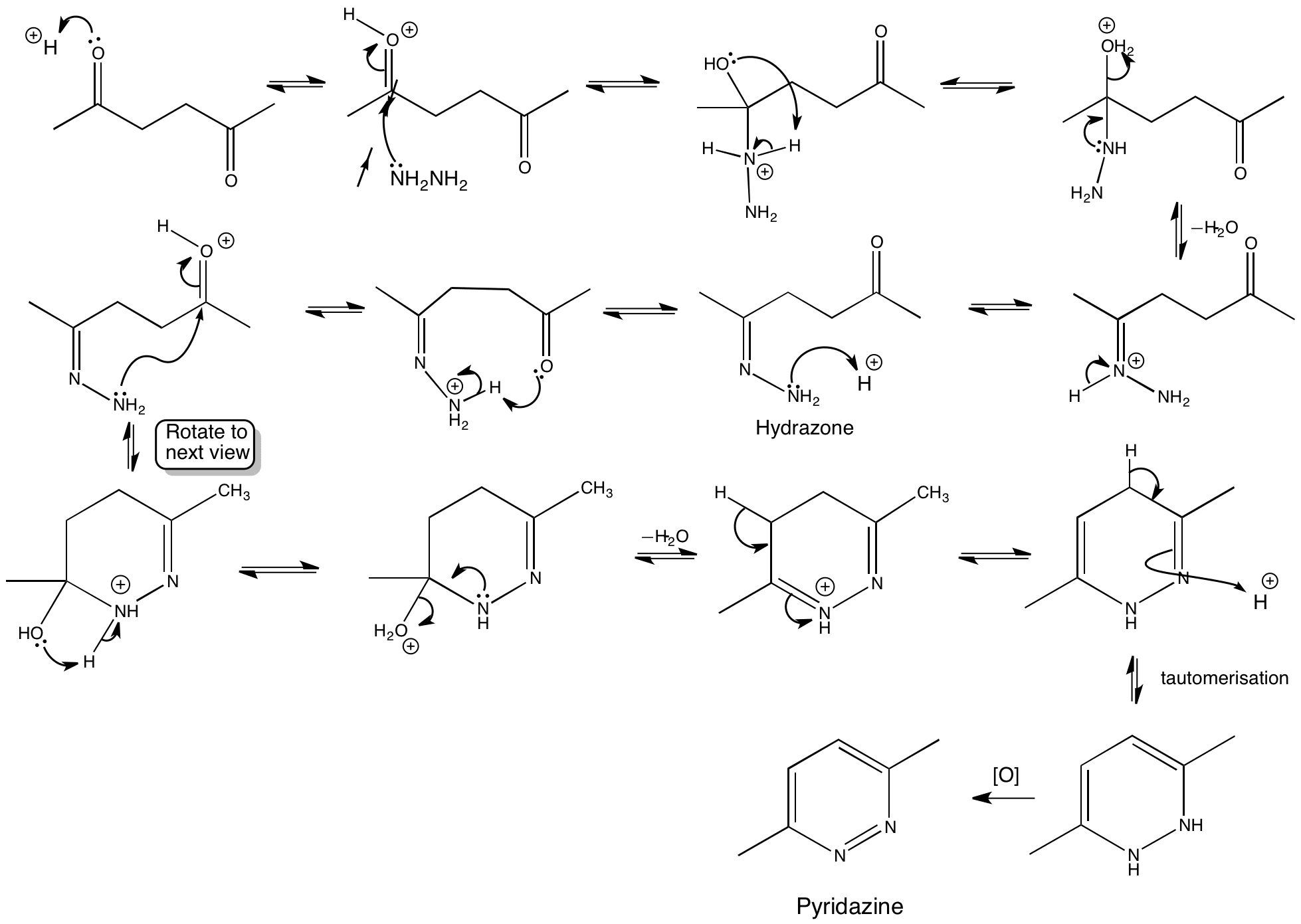
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
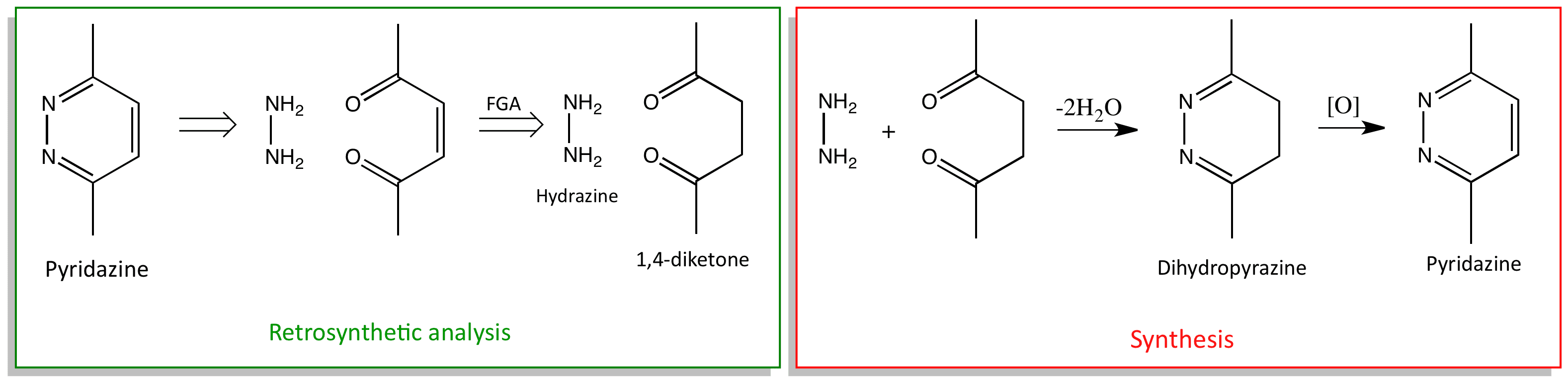
The strategy shown below is adopted in the synthesis is reaction of hydrazine with the 1,4-diketone to give dihydropyridazine. Oxidation of the dihydropyridazine then gives the pyridazine. Carrying out oxidation at this point avoids a cis double bond problem.
Disconnection of pyridazines gives a molecule of hydrazine, and a 1,4-diketone with an alkene in the 2,3-position. A functional group addition , FGA, disconnection gives us the 1,4-diketone.
C. G. Overberger, N. R. Byrd and R. B. Mesrobian, J. Am. Chem. Soc., 1956, 78, 1961–1965.