Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
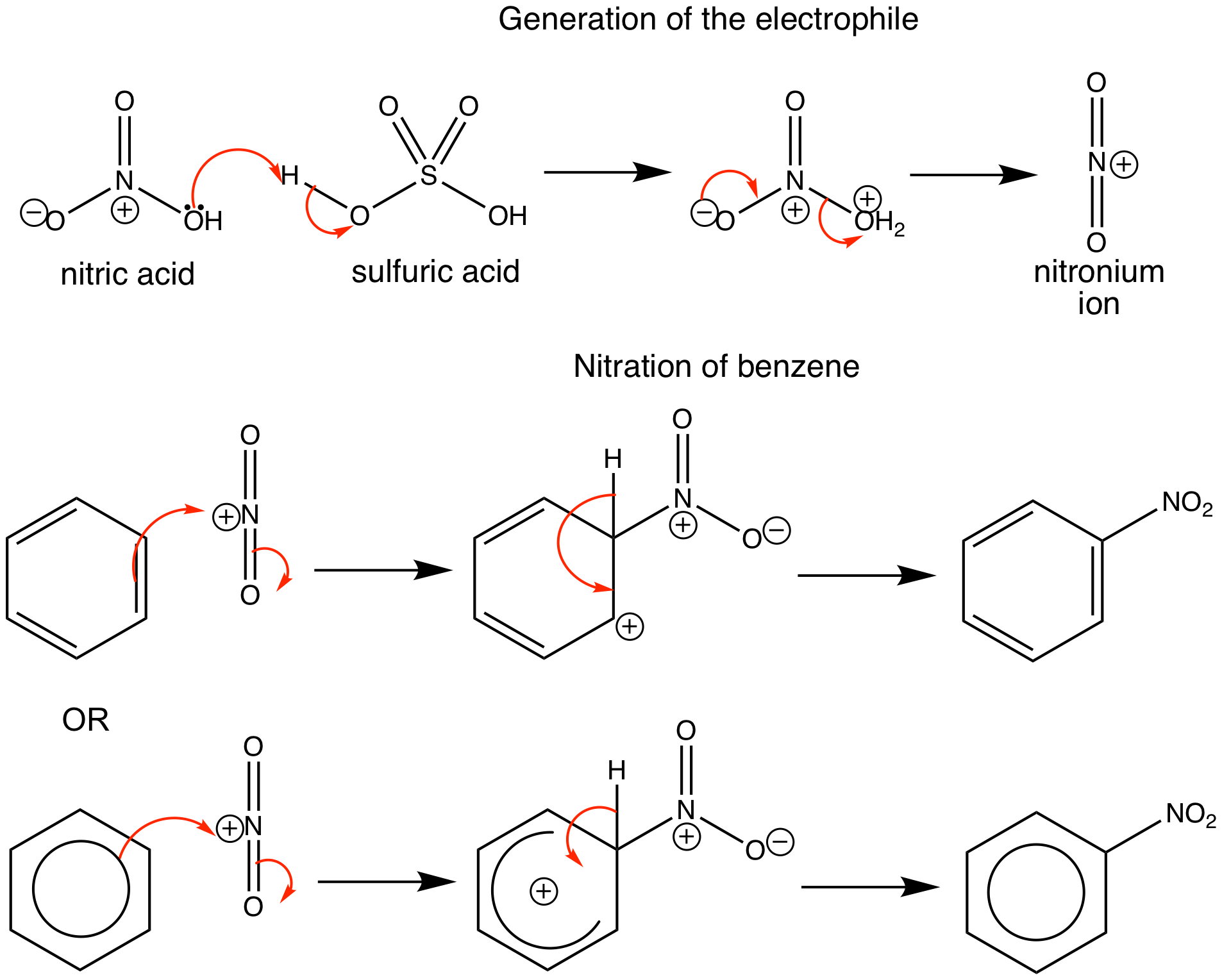
Nitration of benzene firstly involves the formation of a very powerful electrophile, the nitronium ion, which is linear. This occurs following the interaction of two strong acids, sulfuric and nitric acid. Sulfuric acid is the stronger and it protonates the nitric acid on the OH group so that a molecule of water can leave. Benzene attacks the positively charged nitrogen atom of the electrophile, where one of the N=O bonds is broken at the same time. This is followed by rapid loss of a proton to regenerate the aromaticity.