Reaction of Benzyne and Anthracene
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
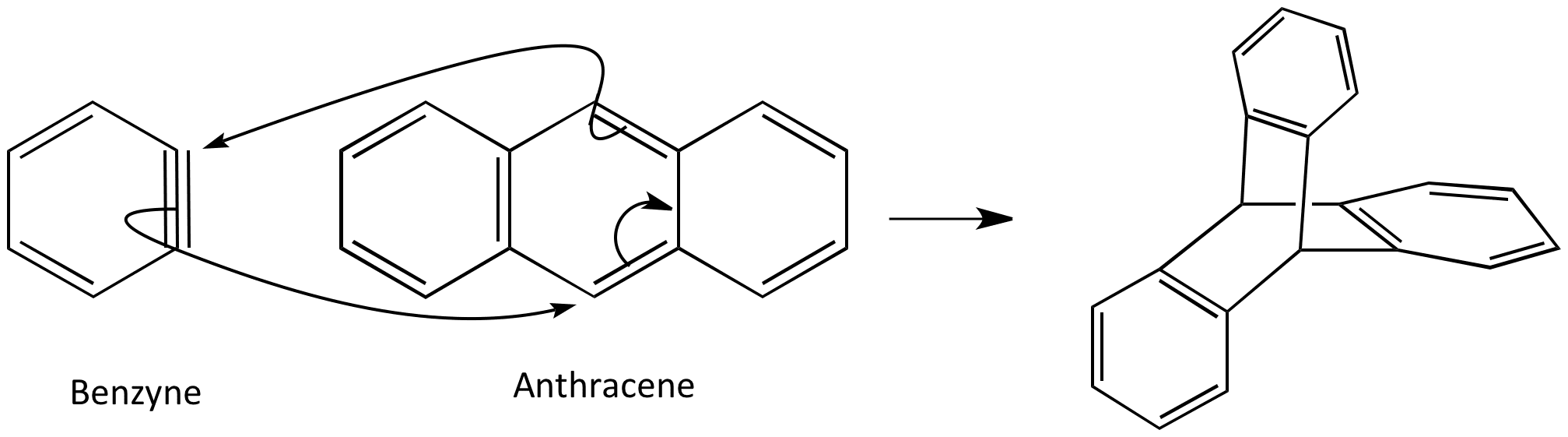
Although the structure of benzyne looks unconvincing, evidence for its existence is provided by the fact it can be trapped in a Diels-Alder reaction. Benzyne is an unstable electrophilic molecule which makes it good dienophile. This is due to it’s low energy LUMO, which is the π* orbital of the triple bond.
Reaction with anthracene gives a symmetrical cage structure. This is difficult to draw in 2D as the molecules approach each other at right angles.
More information about Benzyne formation
J. C. C. Atherton and S. Jones, Tetrahedron, 2003, 59, 9039–9057.