NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
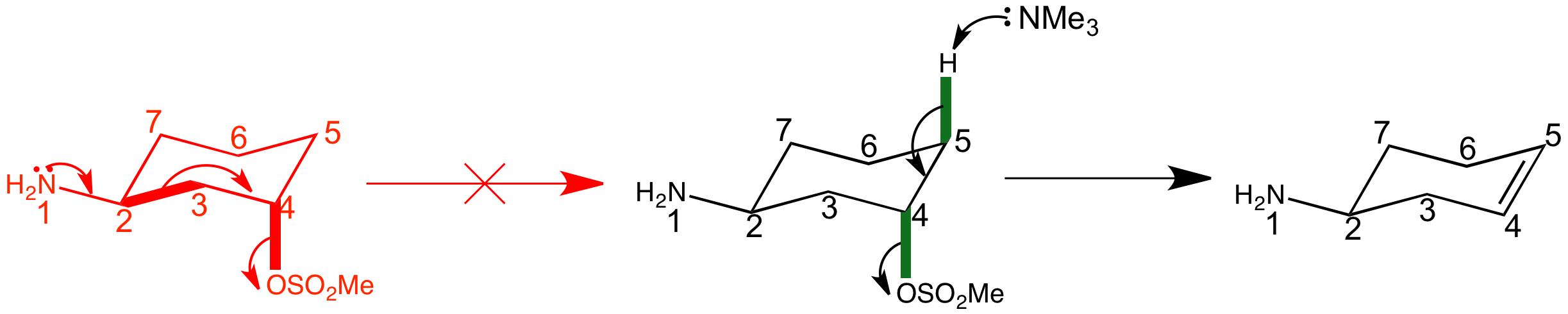
For the trans isomer, fragmentation of the most populated conformation is impossible because the leaving group is not anti-periplanar to any C-C bond. The only bonds anti-periplanar to the mesylate group are C-H bonds (at the 3 and 5 position), making this compound ideally set up for another reaction whose requirements for anti-periplanarity you have already met-E2 elimination.