Click the structures and reaction arrows in sequence to view the 3D models and animations respectively.
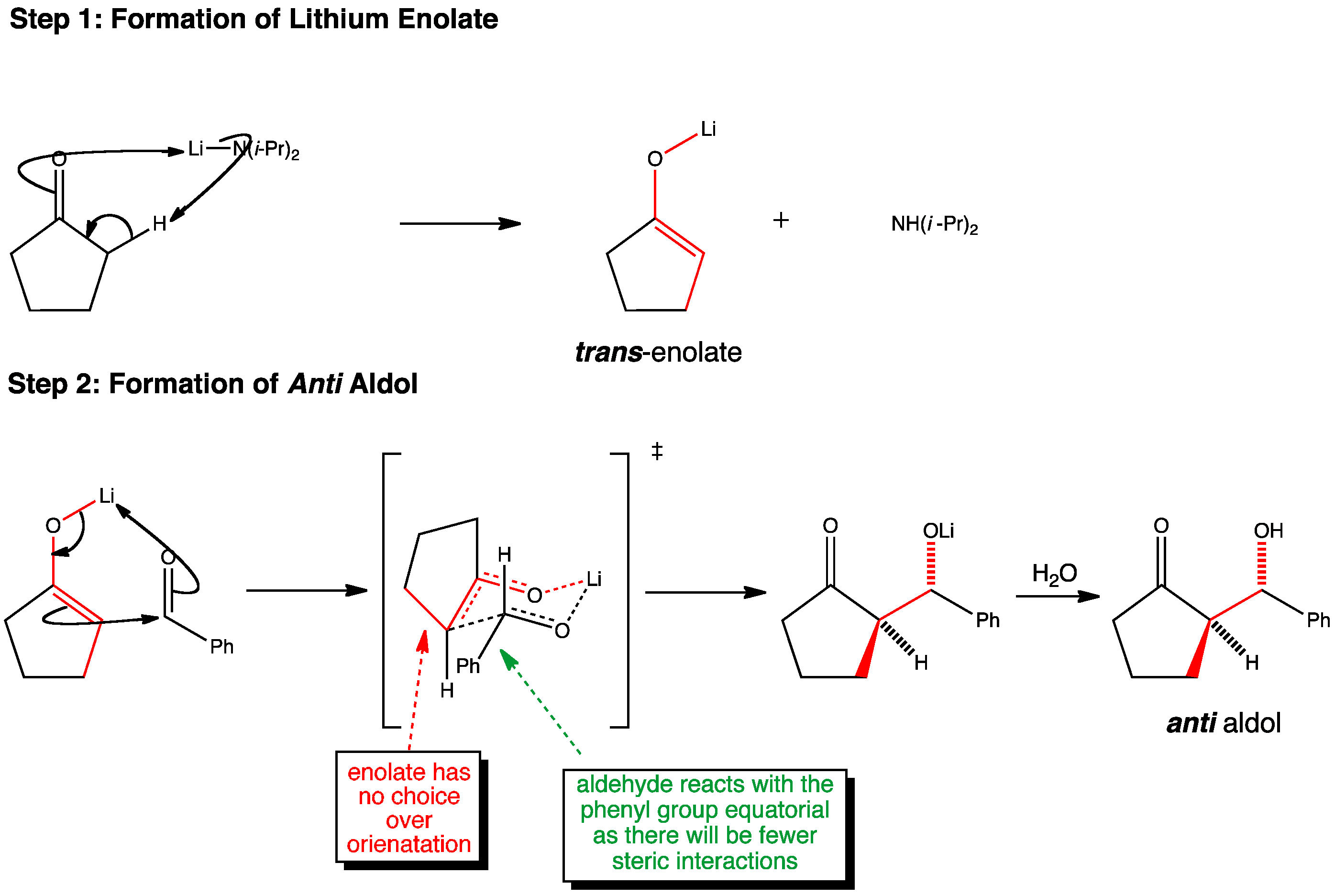
In an aldol reaction, trans enolates prefer to form anti aldols. The reaction proceeds via a chair like transition state where the aldehyde chooses to react with the methyl group equatorial. As the enolate has no choice over its orientation, only an anti product is possible.