Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
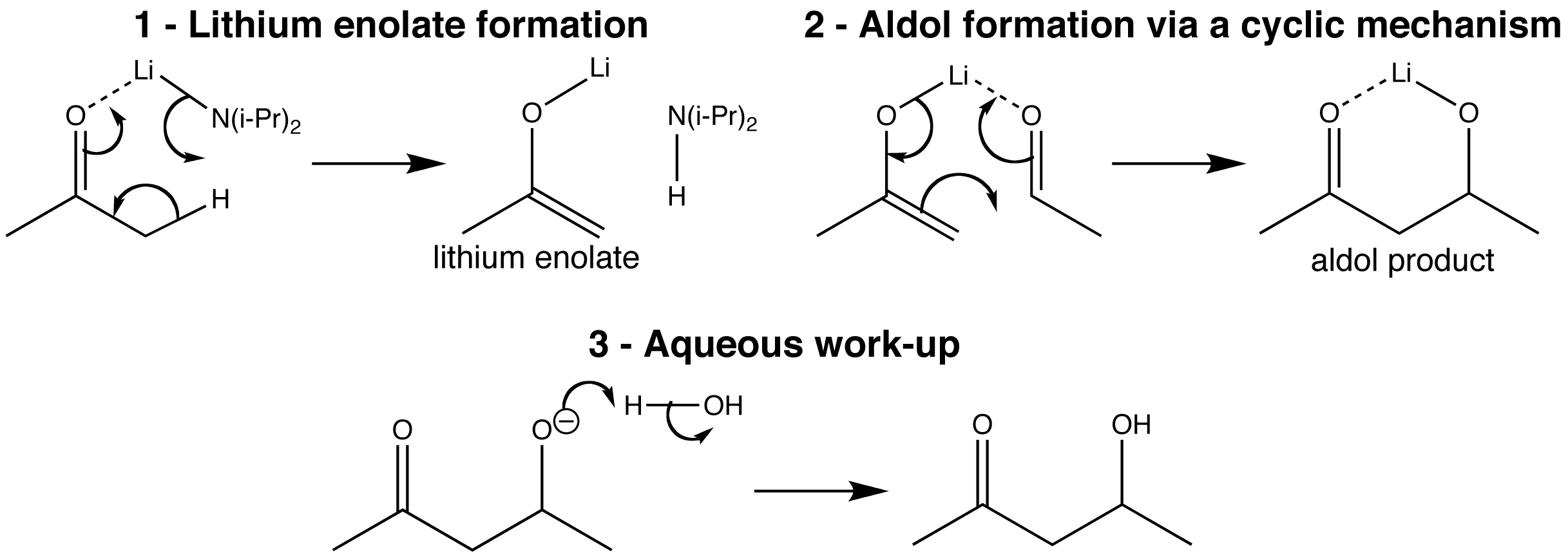
Lithium enolates are formed from the reaction of ketones or esters and LDA at low temperatures. Pre-formed lithium enolates react cleanly with aliphatic aldehydes. That is when a carbonyl compound is added, it complexes with the lithium atom, and allows the aldol reaction to take place by a cyclic mechanism in the coordination sphere of the lithium atom. The product is initially the lithium alkoxide of the aldol, which goes on to give the aldol product after aqueous work-up.