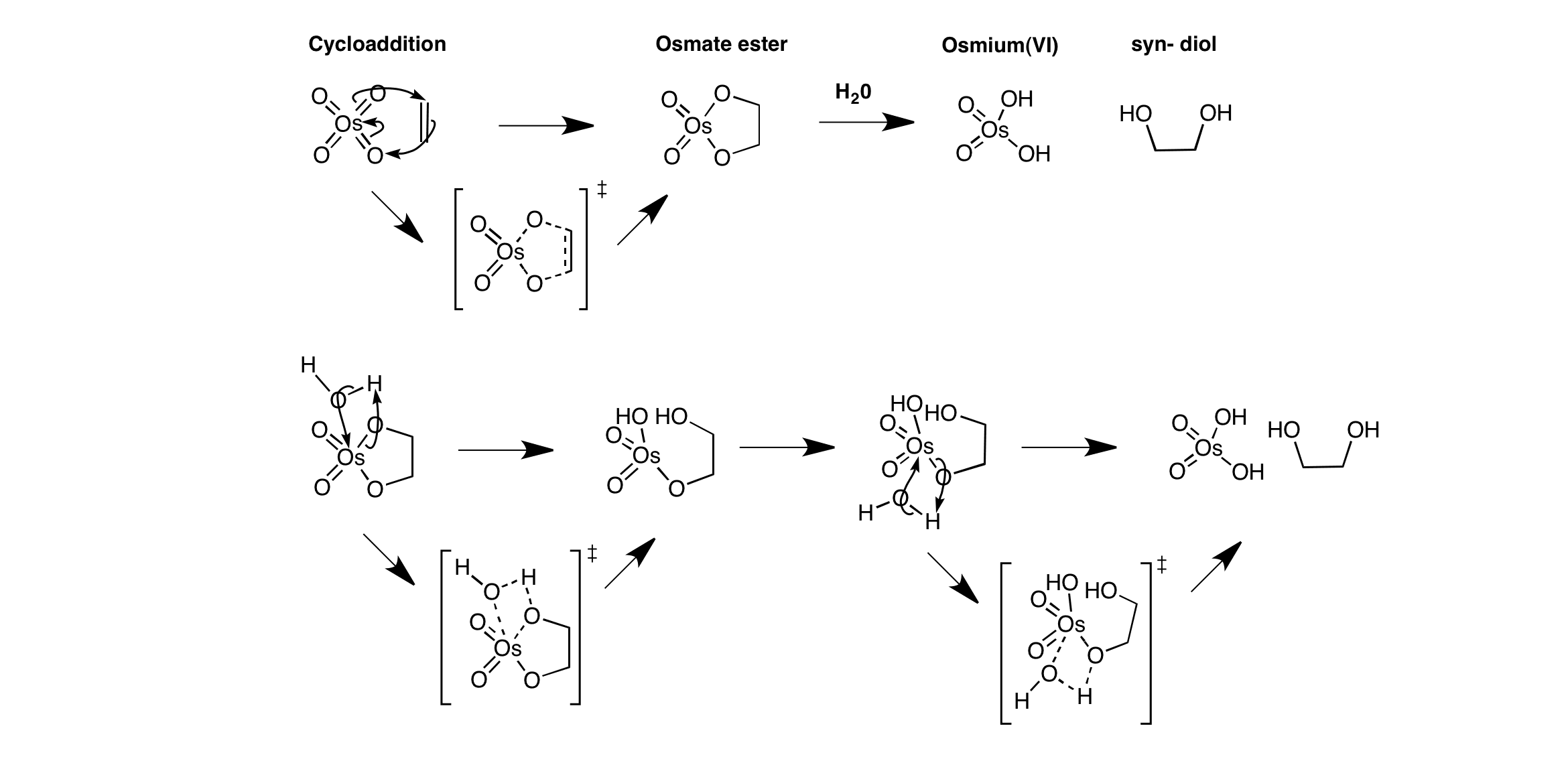
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Syn-dihydroxylation of olefins occur via the reaction with osmium tetroxide. Cycloaddition of osmium tetroxide with ethene proceeds via a cyclic transition state to form an osmate ester. Addition occurs on the same side of the ethene molecule to give a syn-addition. Work-up with water gives the syn-diol and reduced Osmium(VI).
Osmium(VIII) is reduced to Os(VI) over the reaction which is an oxidation.
T. Strassner, in Advances in Physical Organic Chemistry, 2003, vol. 38, pp. 131–160.