NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
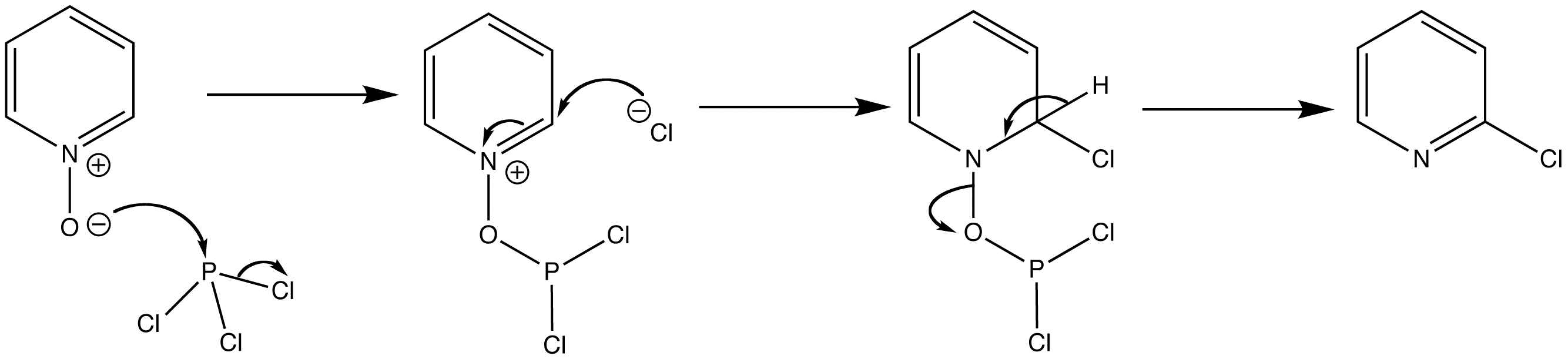
Nucleophilic substitution reactions take place at positions ortho- and para- to the N-oxide. The ususal reagent for this transformation is POCl3 but the mechanism is illustrated with PCl3. Incorporation of the chlorine atom is useful as further nucleophilic substitution reactions can take place at this position.
J.-C. Jung, Y.-J. Jung and O.-S. Park, Synth. Commun., 2001, 31, 2507–2511.