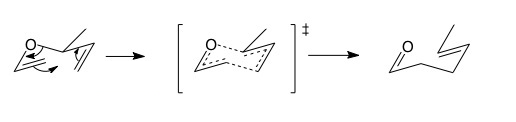
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The Claisen rearrangement is a [3,3]-sigmatropic rearrangement. Count the number of atoms from the old to the new sigma bond and you will see there are 3 atoms in both directions round the ring. [3,3] sigmatropic rearrangements happen though a chair-like transition state. If there is a substituent on the saturated carbon next to the oxygen, then trans (E) geometry is strongly favoured in the product. This a consequence of the chair transition state favouring equatorial substituents and the concerted nature of pericyclic reactions.
Richard Windsor – Undergraduate Final Year Project 2008
J. Rehbein and M. Hiersemann, Synthesis (Stuttg)., 2013, 45, 1121–1159.