Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
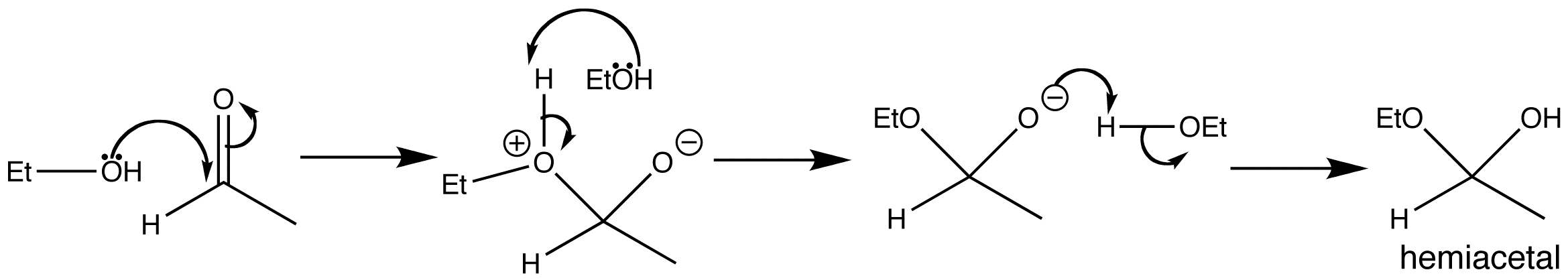
Since water adds to some carbonyl groups, it should come as no surprise that alcohols do too. The product of these reactions is known as a hemiacetal, because it is halfway to the acetal functional group.
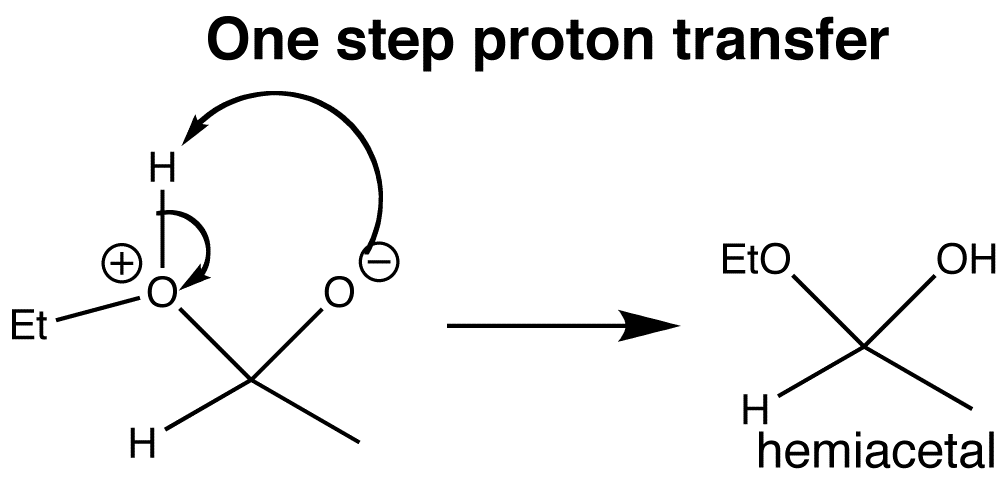
There is no overall consumption of ethanol in the protonation/deprotonation steps, and the order in which these steps happen is not important. It would even be reasonable to write them in one step without involving the alcohol. The addition step is what is important.
G. W. Meadows and B. D. Darwent, Can. J. Chem., 1952, 30, 501–506.