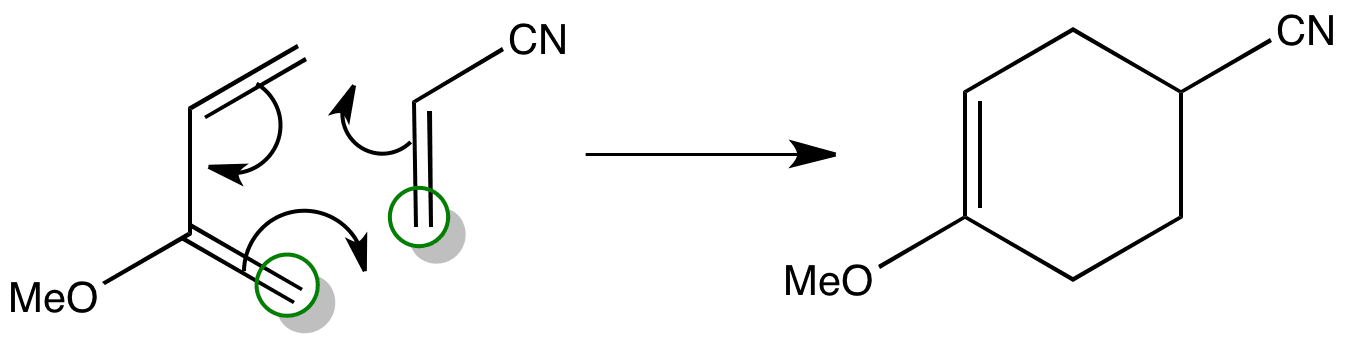
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
It is possible to tell which product is formed by considering the molecular orbitals of the reactants.
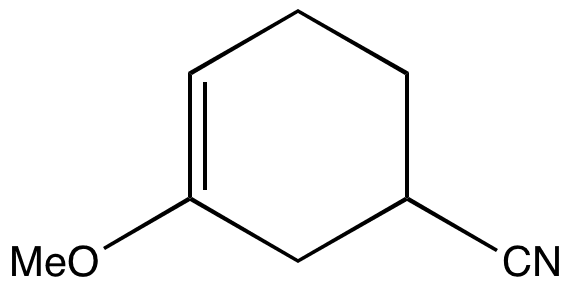
The regioselectivity of the reaction can be determined by working out where the largest component of the HOMO lies in the diene and the largest component of the LUMO lies in the dienophile.
View the molecular orbitals that control the regioselectivity of the Diels-Alder reaction.