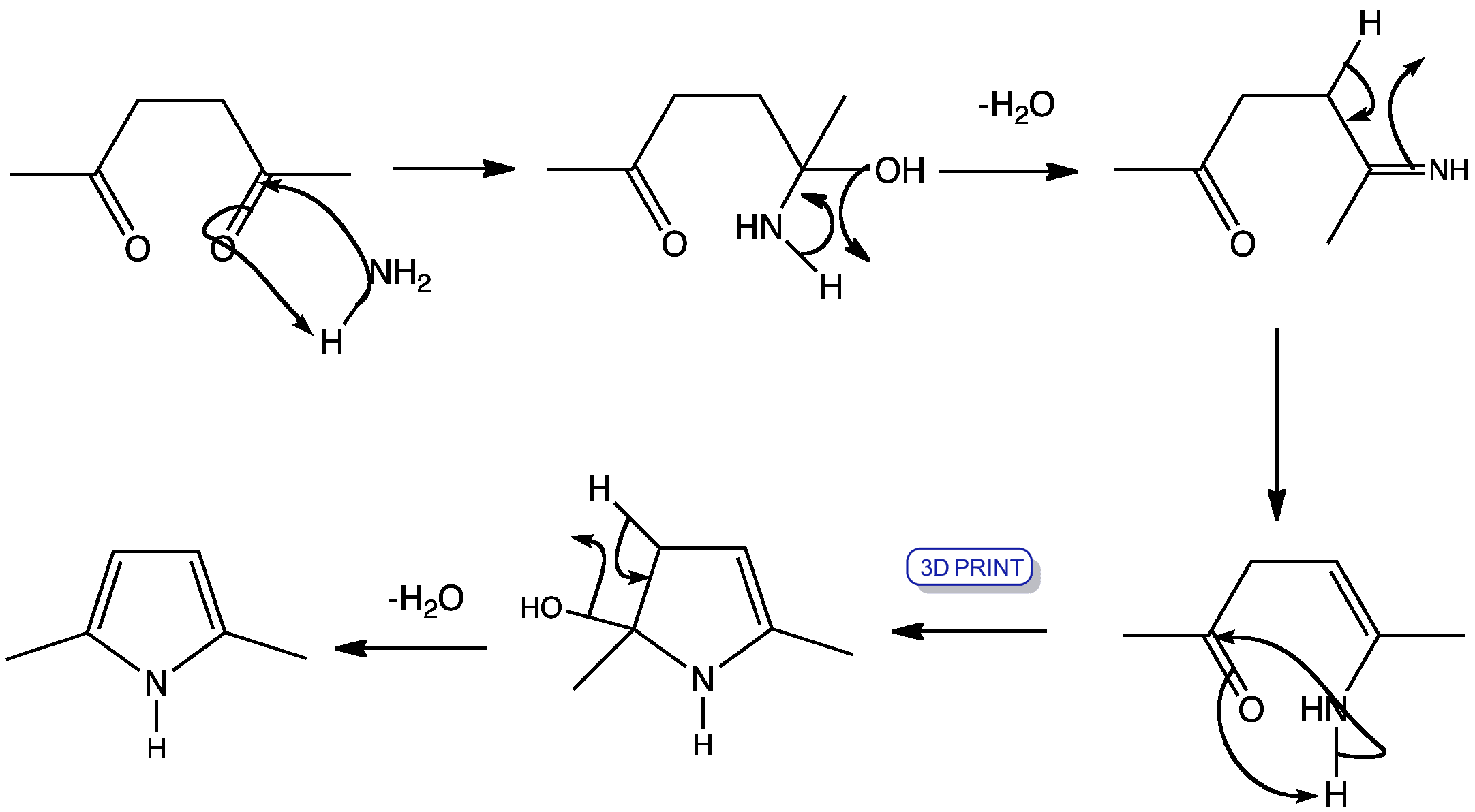
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
This shows the tradtional mechanism for forming a pyrrole ring using a 1,4-diketone and ammonia. The hydroxyl groups are removed as water in two separate dehydration steps, forming enamines in two of the intermediates.
A new mechanism, which does not require enamine formation, has been proposed based on a study using Density Functional Theory.
A. R. Katritzky, D. L. Ostercamp and T. I. Yousaf, Tetrahedron, 1987, 43, 5171–5186.