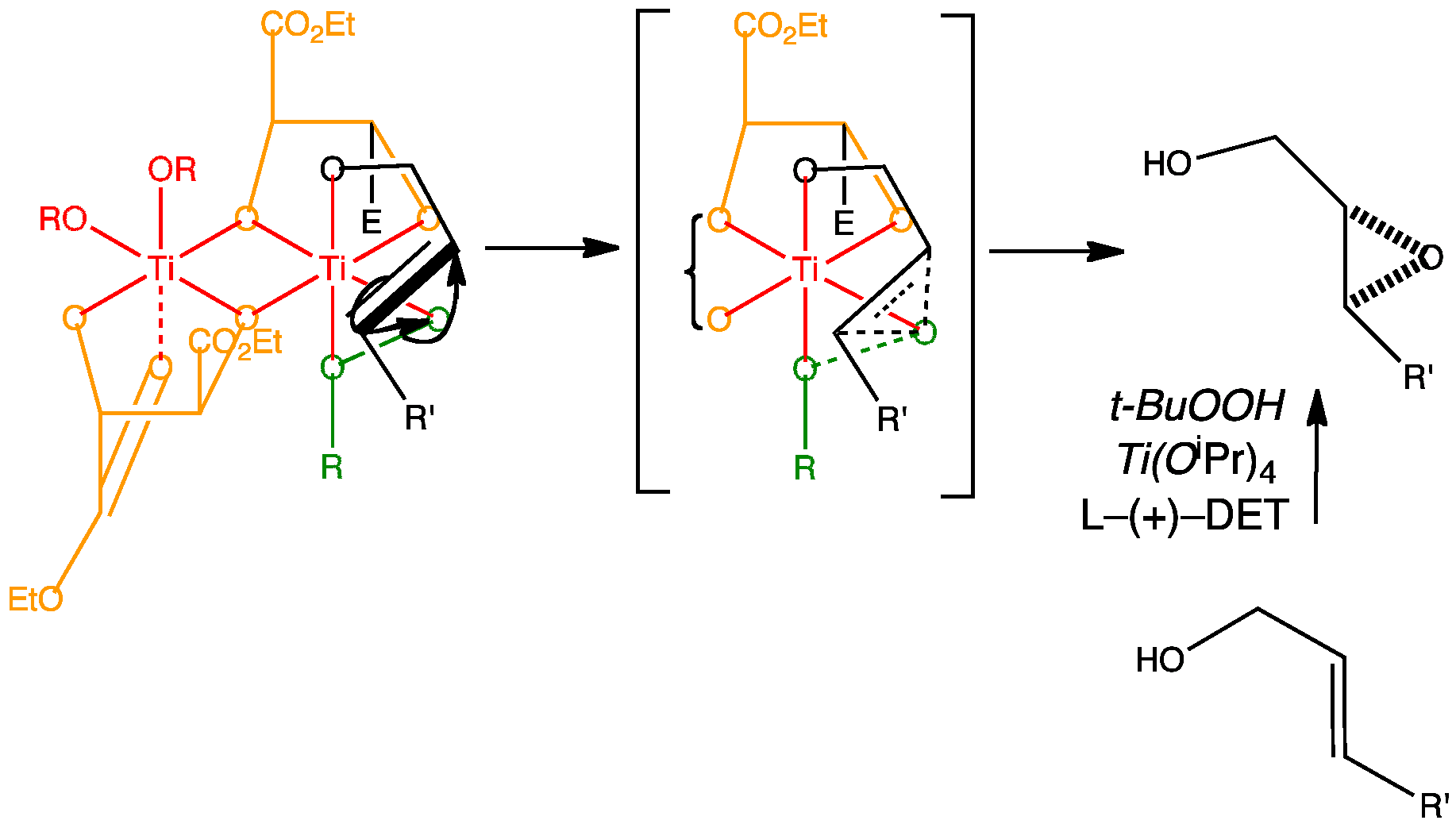
The first of Sharpless’s reactions is the oxidation of an alkene by asymmetric epoxidation. By adding a chiral ligand to the titanium catalyst the reaction becomes asymmetric. The ligand that works best is L-(+)-diethyl tartrate. The active complex is believed to be two titanium atoms bridged by two tartrate ligands.
Methyl esters have been used in the animation for simplicity.
D. J. Berrisford, C. Bolm and K. B. Sharpless, Angew. Chemie Int. Ed. English, 1995, 34, 1059–1070.