Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
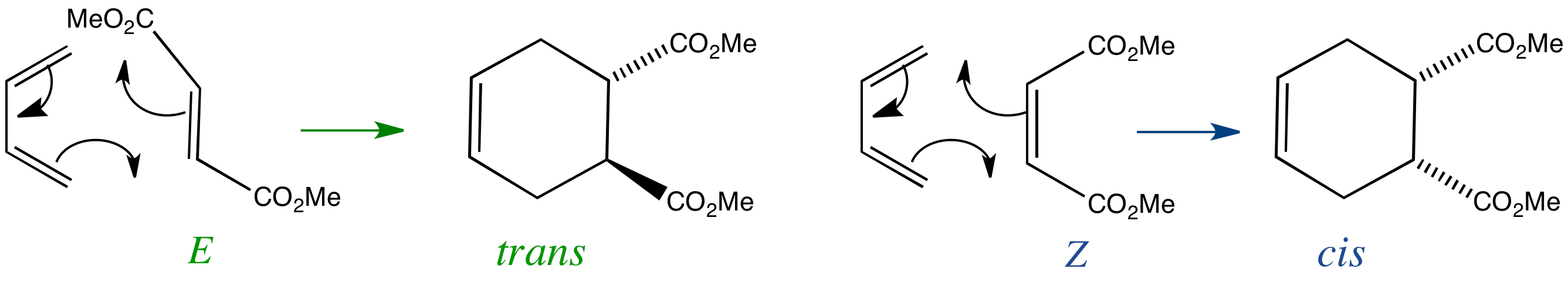
Butadiene undergoes a Diels-Alder reaction with Z or E isomers of unsaturated diesters. The Z isomer is known as dimethyl maleate, and the E isomer is known as dimethyl fumarate.
If substituents are on the same side of the double bond of the dienophile they will be on the same side of the product ring.
If substituents are on opposite sides of the double bond of the dienophile they will be on opposite sides of the product ring.
Substituents which are trans, or E, in the starting dienophile are trans in the product.
Substituents which are cis, or Z, in the starting dienophile are cis in the product.