Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
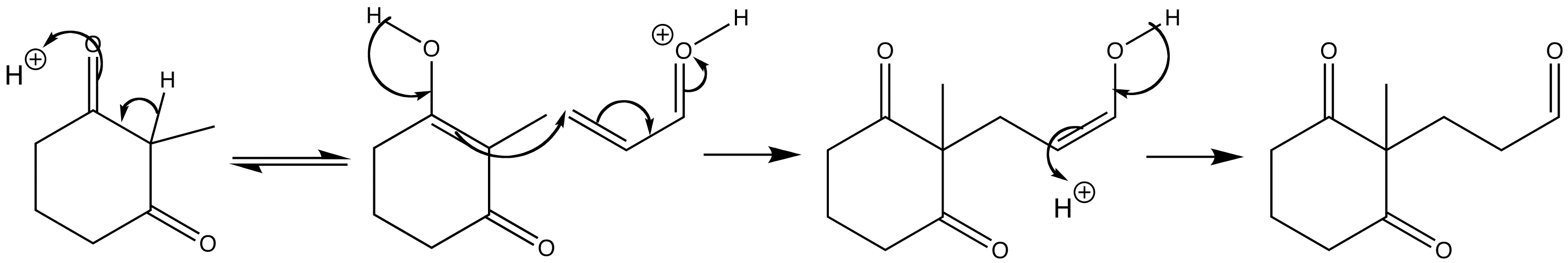
If the nucleophile is sufficiently enolized under the reaction conditions then the enol form is perfectly able to attack the unsaturated carbonyl compound. Enols are neutral and thus soft nucleophiles favouring conjugate attack, and β-dicarbonyl compounds are enolized to a significant extent. The reaction is acid-catalysed.
The mechanism involves acid-catalysed conversion of the keto form of the cyclic β-diketone into the enol form, which is able to attack the protonated enone. The product is the enol form of the triketone, which rapidly tautomerizes to the more stable keto form.
J. Ou, W.-T. Wong and P. Chiu, Org. Biomol. Chem., 2012, 10, 5971.