The meaning of conrotation
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
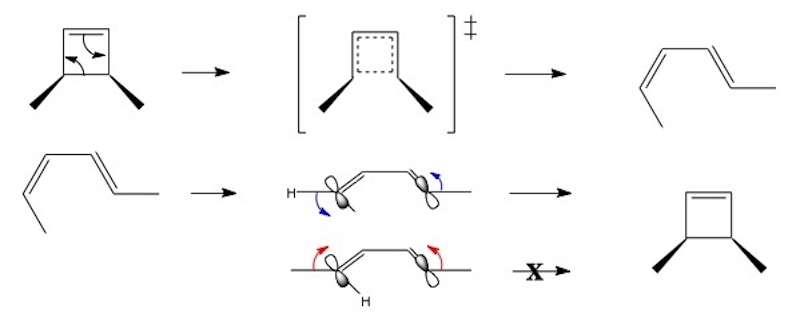
An explanation for the stereospecific nature of electrocyclic reactions can be seen from the reverse reaction.
Both end groups of the Z,E isomer must rotate in the same direction to obtain the required product. This same sense rotation (conrotatory, both anticlockwise above) leads to an in-phase overlap of the terminal orbitals. For the E,E case, the terminal groups must rotate in an opposite sense.
This is the crucial idea: such motion would lead to unfavourable antibonding overlap.
Richard Windsor – Undergraduate Final Year Project 2008
A. Misale, S. Niyomchon and N. Maulide, Acc. Chem. Res., 2016, 49, 2444–2458.