Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
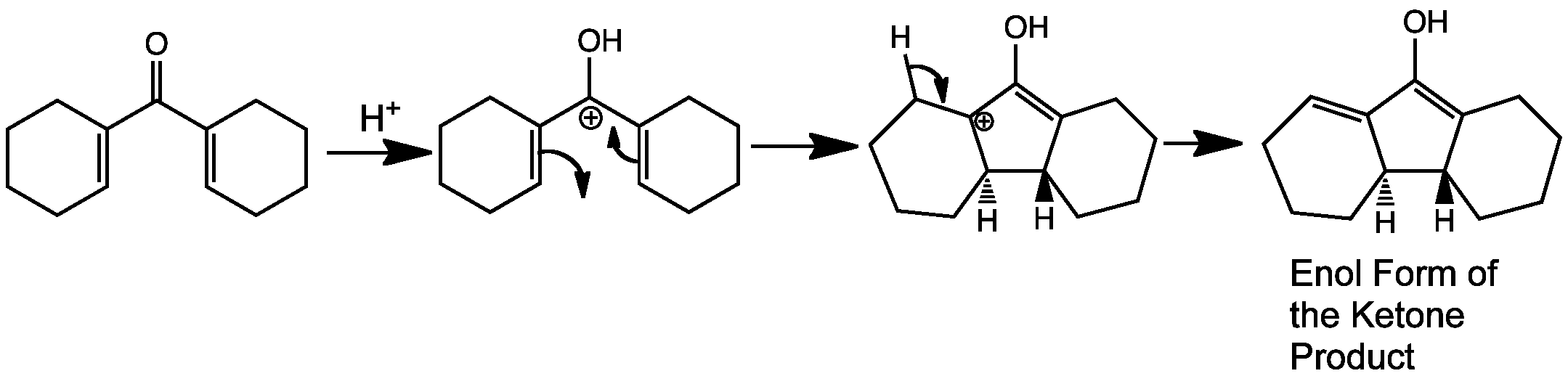
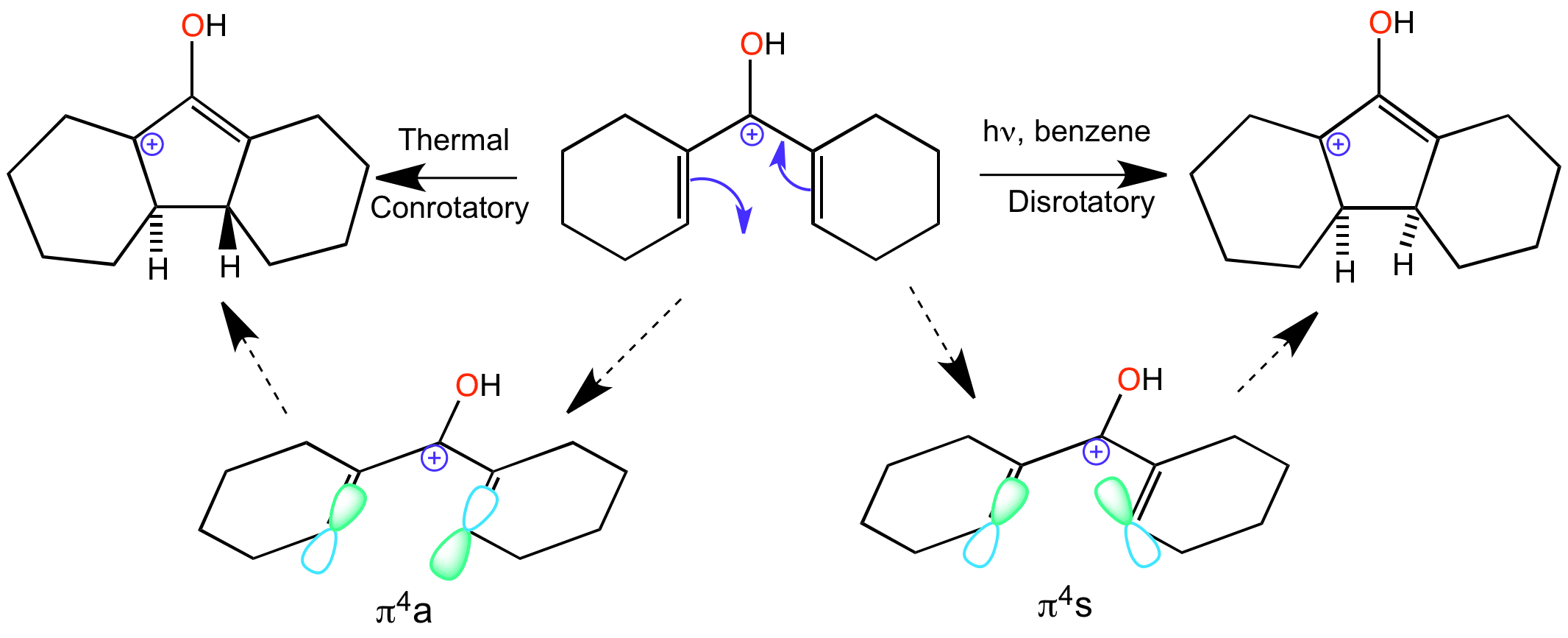
The Nazarov cyclization is an electrocyclic reaction which involves ring closure of a doubly alpha beta unsaturated ketone to a cyclopentenone. This is carried out by initial protonation of the carbonyl group leading to formation of the 4 pi system delocalised over 5 carbons atoms – one of the p-orbitals in the system is empty.
The directionality of the ring closure depends on the conditions of the reaction. Under thermal conditions the reaction goes via a conrotatory motion, the product ends up with the two hydrogens on opposite faces, one up one down. Disrotatory under these conditions is not allowed because it’s a 4pi-system This is the reaction that is animated on this page.
However under photochemical conditions disrotatory motion is allowed, which means that the product has both of the hydrogens on the same face, either both up or both down.
Clicking on the word “conrotatory” shows the movement of the orbitals as part of the animation of the reaction.
Final Year Project 2013: Ilona Blee
A. J. Frontier and C. Collison, Tetrahedron, 2005, 61, 7577–7606.