Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
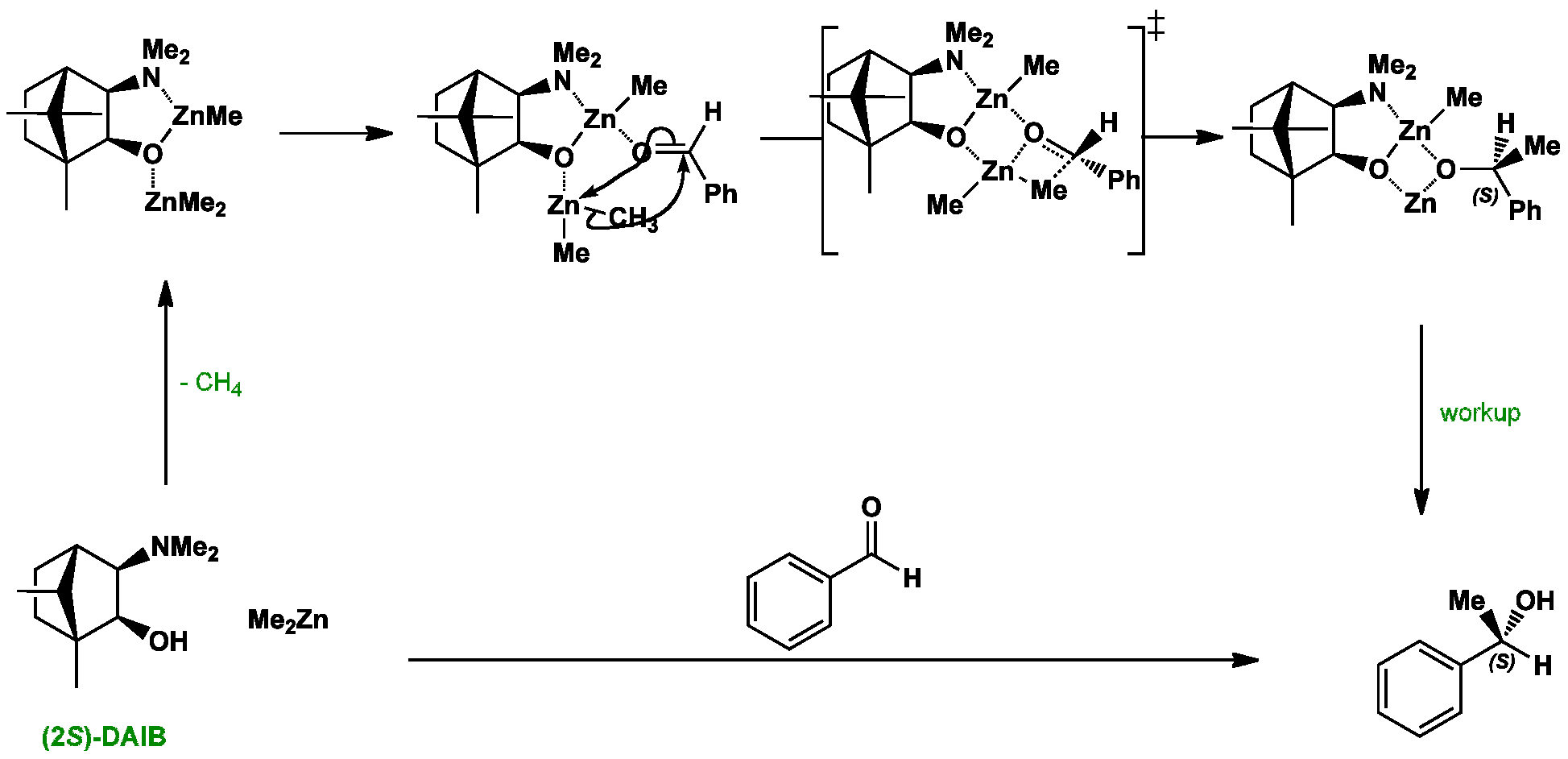
Rigid bicyclic amino alcohols such as DIAB catalyse the addition of dimethyl zinc to aldehydes. Initial reaction with one organozinc produces a bimetallic intermediate. This then coordinates with the aldehyde so that the bulkier group (in this case, Ph) occupies the less hindered position. Intramolecular delivery of the methyl leads to the S-enantiomer, which is released on workup.
M. Yamakawa and R. Noyori, Organometallics, 1999, 18, 128–133.