 Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
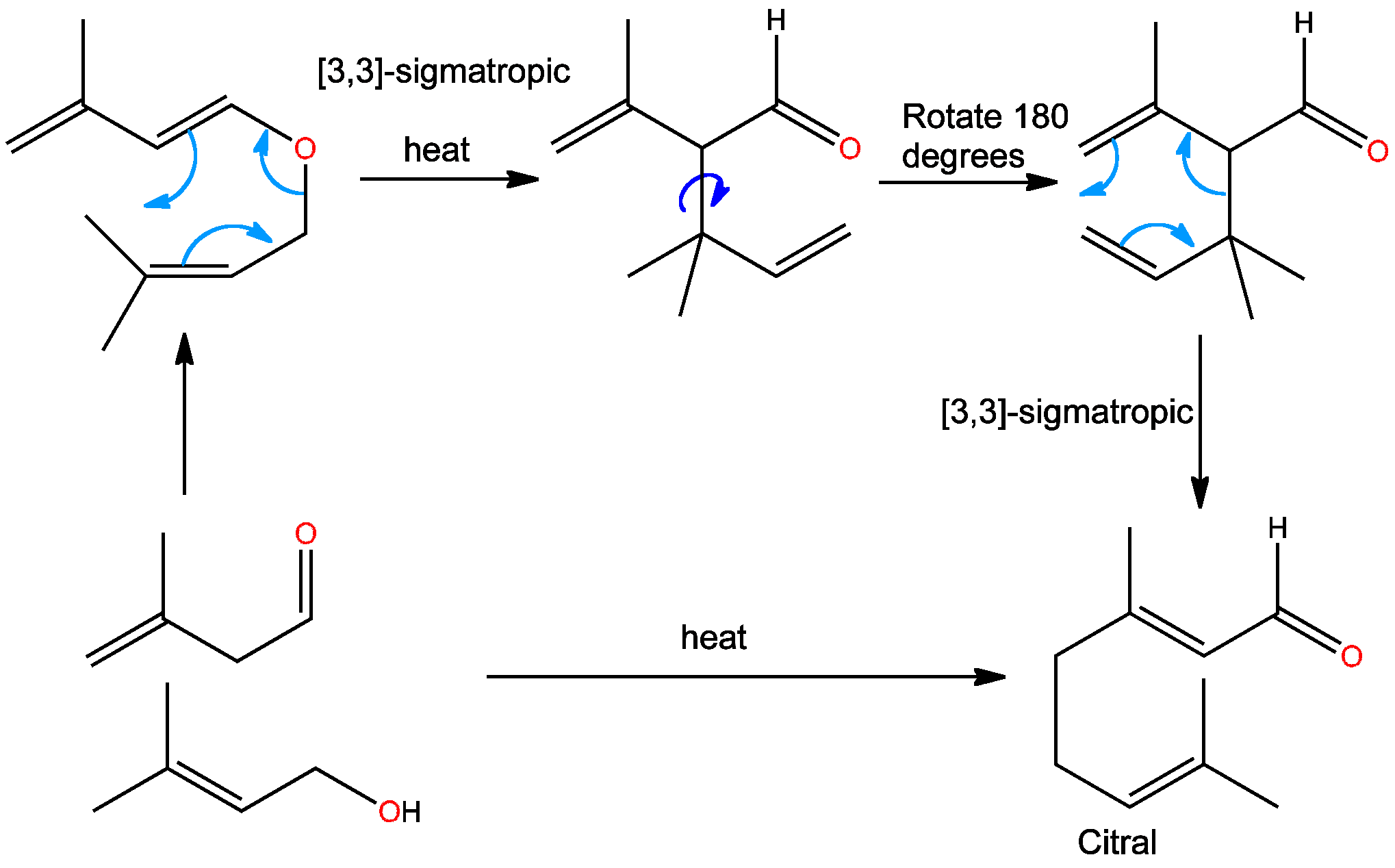
Click the structures and reaction arrows in sequence to view the 3D models and animations respectivelyCitral is a compound which can be used in the synthesis of vitamin A. This industrial synthesis is made up of two different [3,3]-sigmatropic rearrangements done in sequence.
The first is a Claisen rearrangement which involves an oxygen in the chair-like transition state. More details of the Claisen rearrangement can be found here. The second part of the single step reaction is a Cope-rearrangement, in which only carbons are involved in the chair-like transition state. These two reactions happen in succession when the two starting materials are heated together, and the reaction is driven by formation of a conjugated carbonyl group in the product.
Final Year Project 2013: Ilona Blee