SF4 Sulfur Tetrafluoride
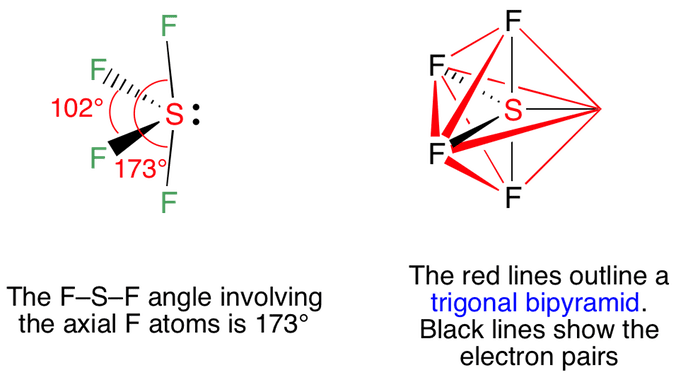
Sulfur tetrafluoride has 5 regions of electron density around the central sulfur atom (4 bonds and one lone pair). These are arranged in a trigonal bipyramidal shape with 102° F-S-F bond angles between the equatorial fluorine atoms and 173° between the axial fluorine atoms.
The lone pair takes an equatorial position because it demands more space than the bonds. The result is a disphenoidal or ‘see-saw’ shaped molecule.
Click the structures to load the molecules
Related structures H2O | NH3 | CH4 | PF5 |SF4 |ClF3 | SF6 | XeF4




