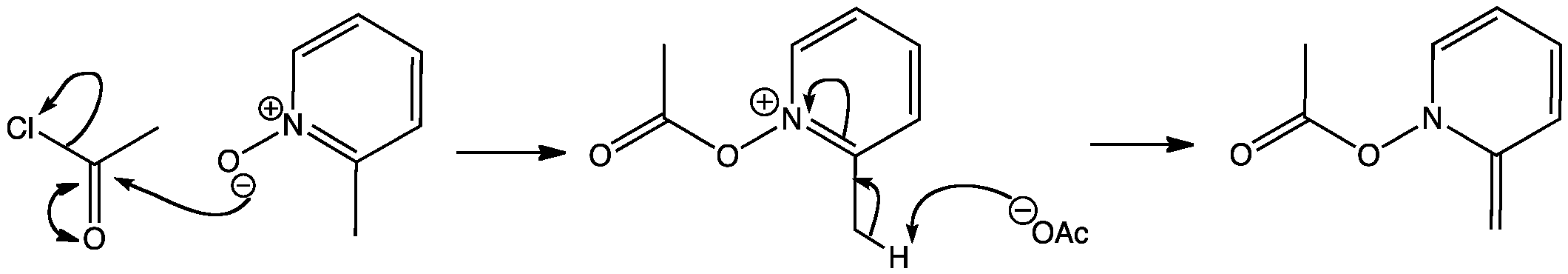
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Nucleophilic addition at distant sites on pyridine N-oxides is possible on reaction with acid anhydrides, if there is an alkyl group in the 2-position. Acylation occurs on the oxygen, then a proton is removed from the side chain to give an uncharged intermediate.
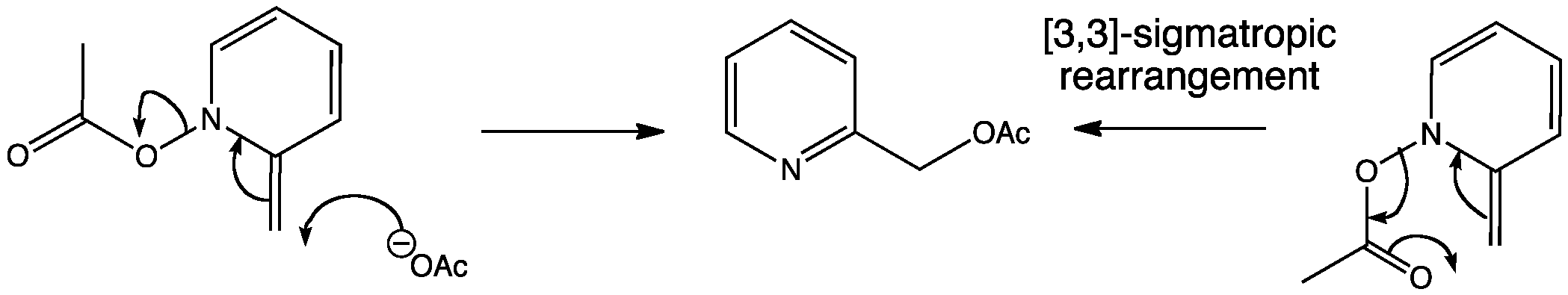
This compound can then either undergo nucleophilic attack, or sigmatropic rearrangment to give the same product. Notice the six-membered tranisition state which is characteristic of a [3,3]-sigmatropic rearrangment.
The reaction is usually carried out with acetic anhydride but acetyl chloride has been used to model the acylation.
S. Oae, S. Tamagaki, T. Negoro and S. Kozuka, Tetrahedron, 1970, 26, 4051–4063.