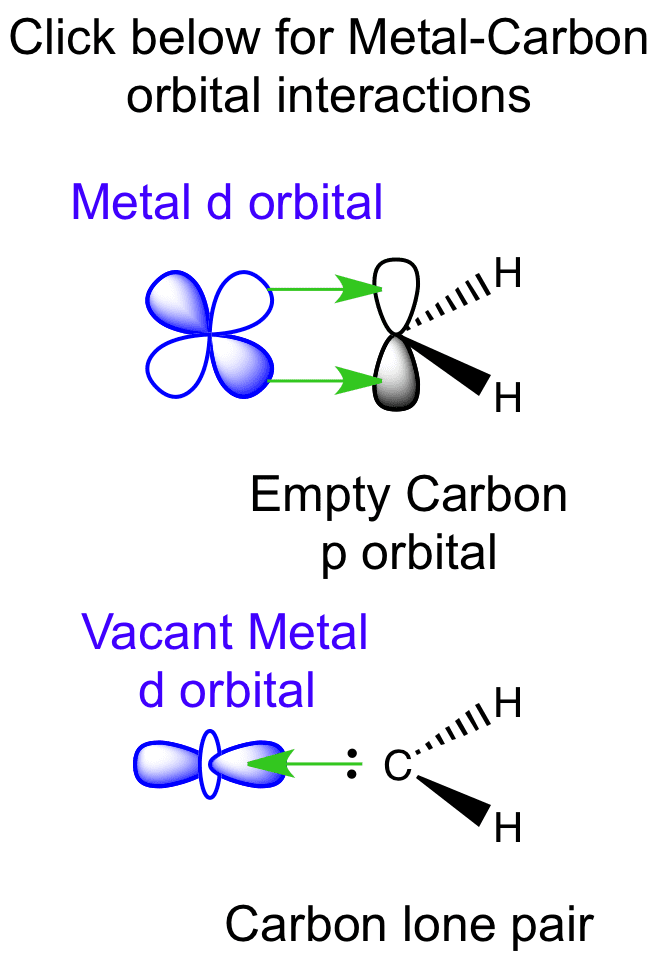
Fischer and Schrock carbenes contain a metal-carbon double bond. The electron pair on carbon is donated to the metal center forming a σ bond. The empty carbon p orbital can accept electron density from the metal, via π backbonding.
Fischer carbenes are electron deficient on the carbon and hence are susceptible to nucleophilic attack.
Schrock carbenes are electron rich on the carbon due to strong backbonding and hence are susceptible to electrophilic attack.
Explore Metal-Ligand bonding with other molecules
Carbon Monoxide | Phosphine | Hydrogen | Ethylene | Cyclobutadiene | Butadiene | Benzene | Allyl | Cyclopentadienyl | Carbene