Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
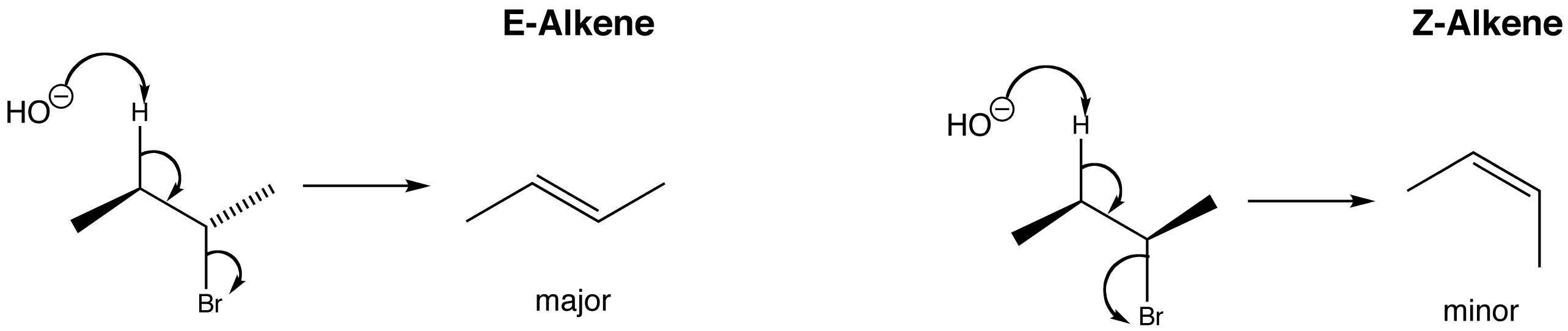
In this elimination reaction, a new double bond is formed when the carbon – bromine bond breaks at the same time as the –OH removes the H+. The elimination takes place from the anti-periplanar conformation, as this is the most stable conformation.
As you can see from the reaction above, when 2-bromobutane undergoes an elimination reaction, two possible stereoisomers are formed. This is because 2-bromobutane has two conformations with H and Br anti-periplanar, but the one that is less hindered forms the major product, so the E-alkene predominates.