Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
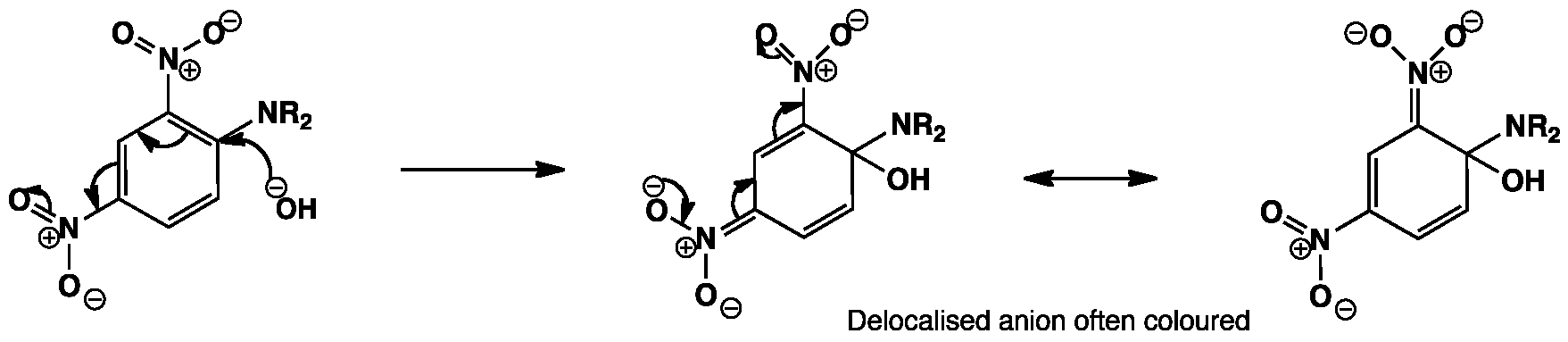
The hydroxy group adds into the aromatic ring at the position para to one of the nitro groups and the negative charge is stabilized by the nitro group. The additional nitro group ortho to the attack allows delocalization of the negative charge in the intermediate which gives rise to the characteristic purple colour of these intermediates.