Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
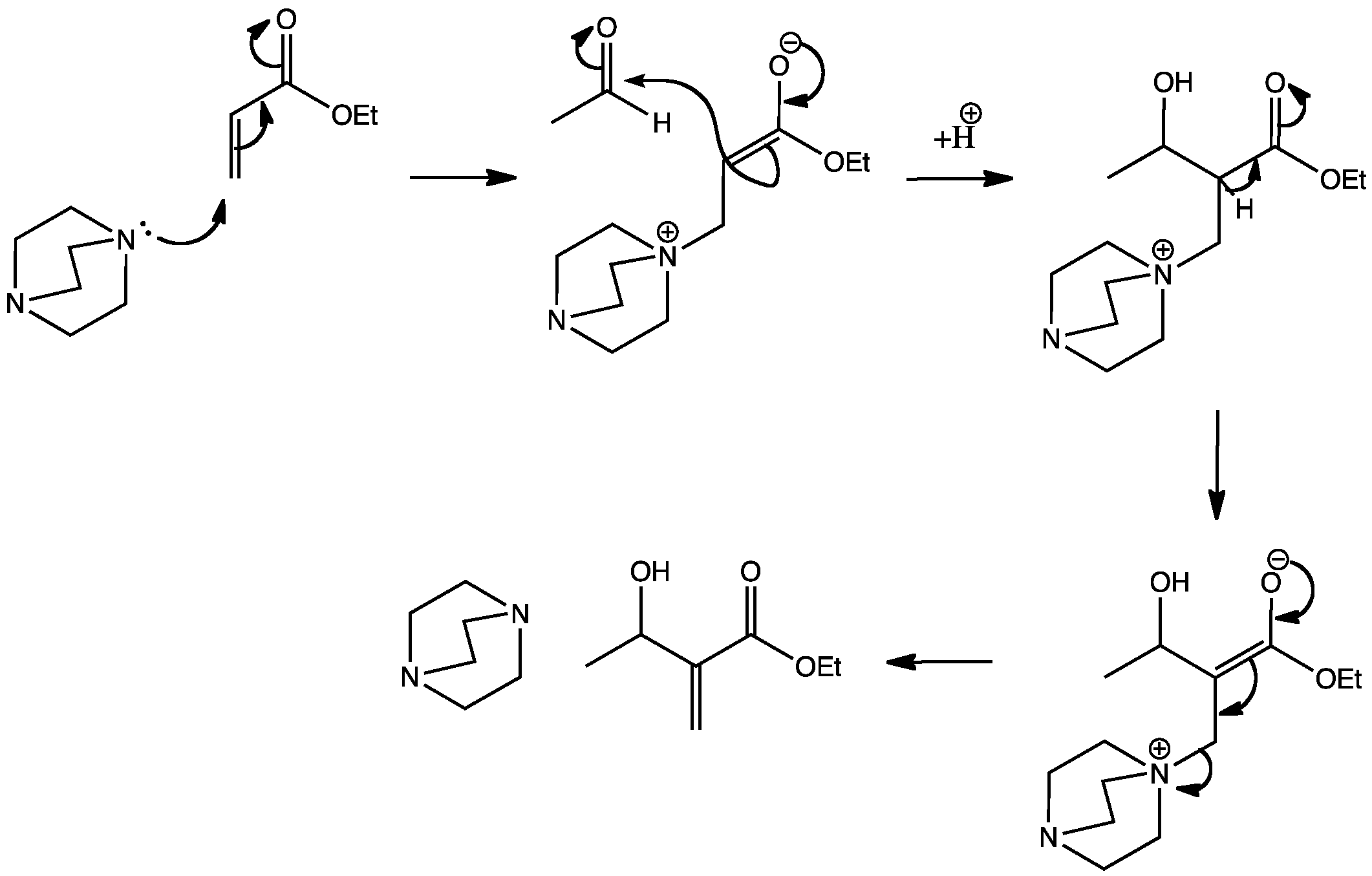
Discovered in 1972, the Baylis-Hillman reaction uses a modified aldol step. Instead of the usual enolate being formed by deprotonation, it is formed by conjugate addition. Nucleophilic 1,4-diazabicyclo[2.2.2]octane (DABCO) undergoes conjugate addition to ethyl acrylate. An enolate is formed which then attacks the acetaldehyde in an aldol reaction. E1cB eliminations often follow aldol reactions, but in this case DABCO is a better leaving group than the hydroxyl group. DABCO is then regenerated as a catalyst.
D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811–892.