Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
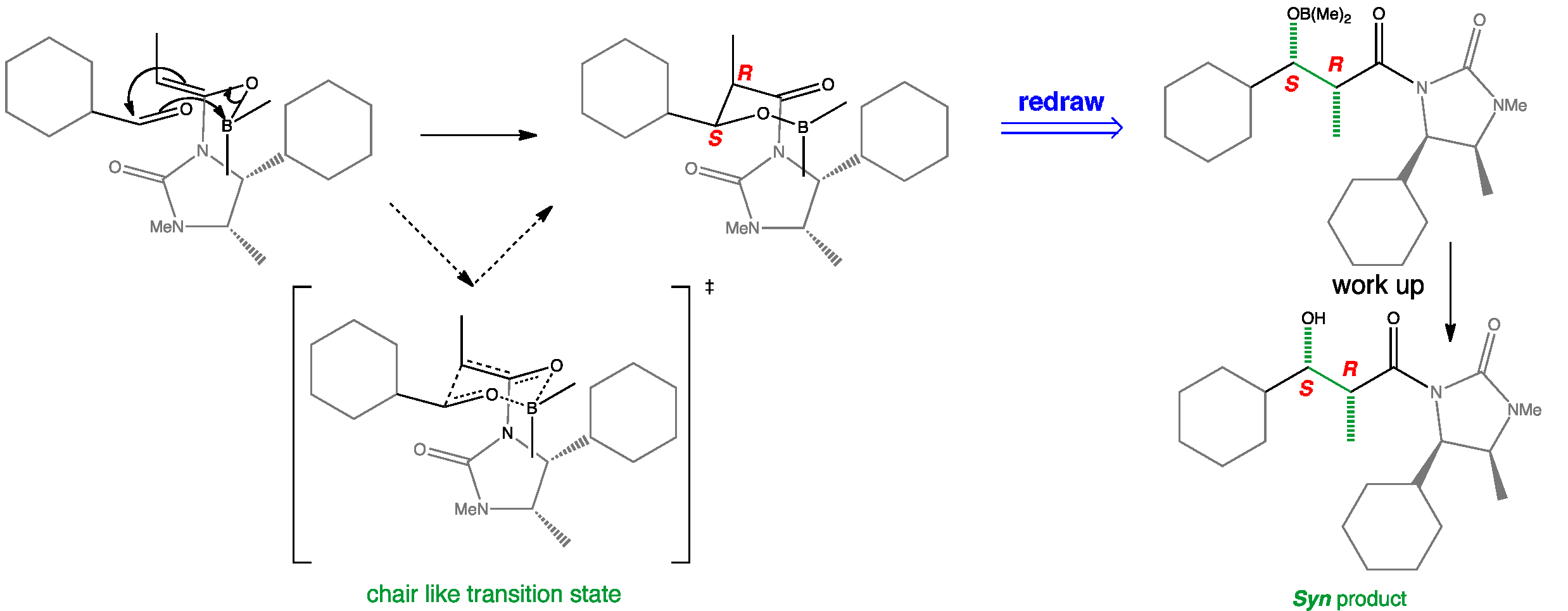
The Z-boron enolate adopts the non-chelated conformation with the imide carbonyl opposite the enolate oxygen. The auxiliary hinders the lower face of the enolate so the aldehyde attacks from above to form a chair like transition state. Boron enolate Z-geometry produces the syn product whereas the auxiliary controls facial selectivity and therefore a single enantiomer is formed.
D. a Evans, J. V Nelson and T. R. Taber, J. Am. Chem. Soc., 1981, 103, 3099–3111.