NOTE: For the electrostatic surfaces red is electron rich, blue is electron poor
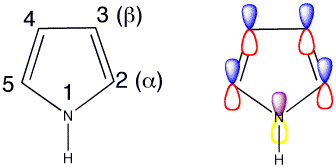
Click the checkboxes to view the atom numbering and p orbitals respectively
The lone pair on nitrogen is in the p orbital so it is involved in the 6 pi-electron aromatic system. Hence pyrrole is not very nucleophilic and is only weakly basic at nitrogen. Looking at the HOMO of pyrrole the lobes are much bigger at the 2- and 5- positions, this indicates that the reactions are most likely to take place at these positions.
Attack at C2 is also most favoured if you consider resonance forms as there are three resonance forms of the cation available, whereas there are only two resonance forms following attack at C3 so attack is less favoured at C3.