Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
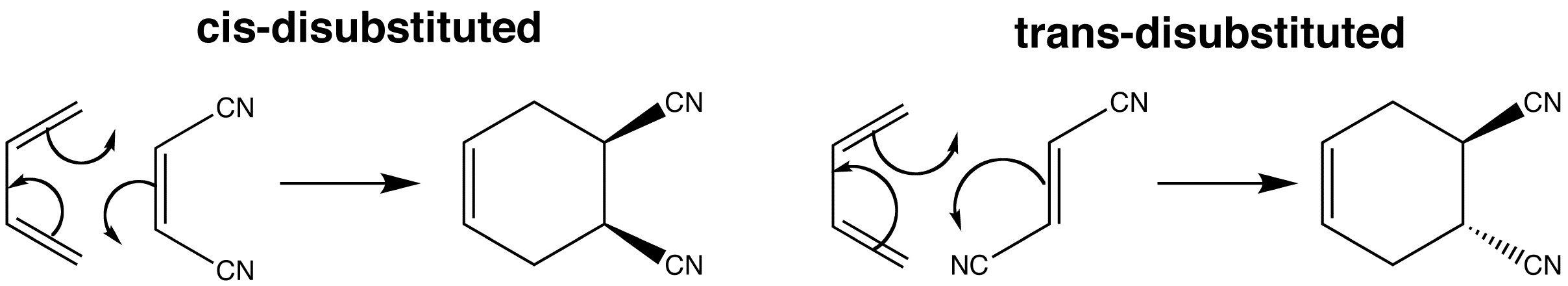
If there is stereochemistry in the dienophile, then it is faithfully reproduced in the product. Thus cis and trans dienophiles give different diastereoisomers of the product. The cyano groups simply stay where they are, that is if they are cis in the dienophile, they remain so in the product and vice versa.
Note that the initial conformation of the products is a boat resulting from the boat transition state of the Diels-Alder reaction.