Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
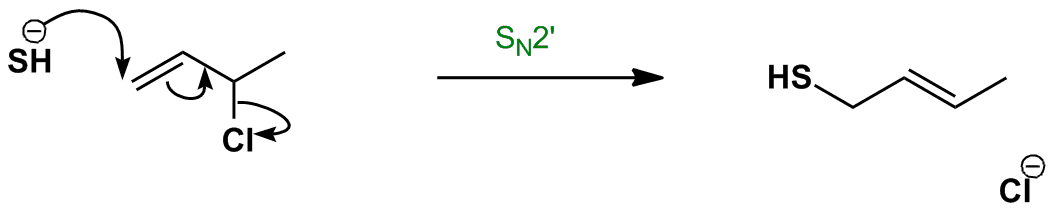
This subclass of nucleophilic substitution occurs when the nucleophile (HS–) attacks the alkene instead of the saturated carbon – the SN2′ mechanism. This is the due to the saturated carbon being hindered (it is a secondary carbon), making the regular SN2 mechanism less favourable.
Alternatively, use these links: (Substrate: Nucleophile)
Allyl chloride : SH | Benzyl chloride : SH | 2o benzyl chloride : SH | 2o allyl chloride : SH (SN2′)
R. H. DeWolfe and W. G. Young, Chem. Rev., 1956, 56, 753–901.
J. W. Hill and A. Fry, J. Am. Chem. Soc., 1962, 84, 2763–2769.