Click the reaction arrow in sequence to view the 3D models and animations respectively
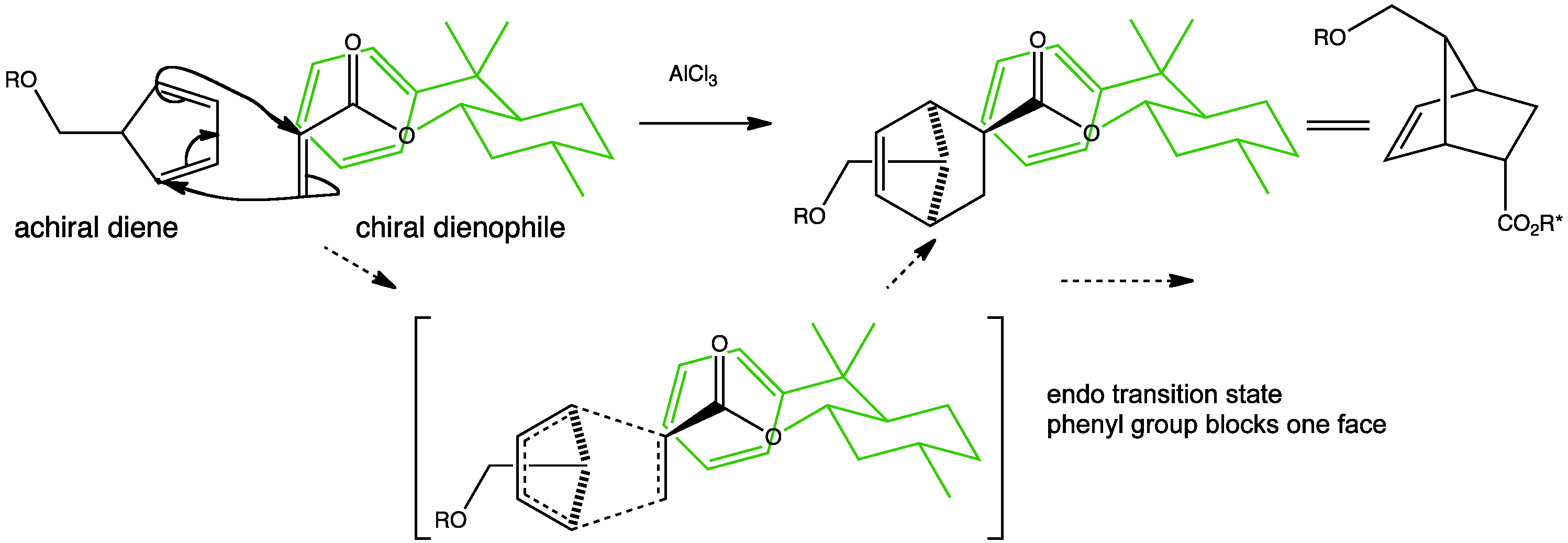
8-Phenylmenthyl esters shield one face of the ester because of the chair conformation of the cyclohexane and the bulky group occupying an equatorial position. Diels-Alder reactions promoted by Lewis acids follow the normal endo selectivity and in this example the substituent on the cyclopentadiene prefers to occupy the less hindered face. The adduct was used by Corey to make prostaglandins.
E. J. Corey and H. E. Ensley, J. Am. Chem. Soc., 1975, 97, 6908–6909.