NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition state phase.
Click the structures and reaction black arrows in sequence to view the 3D models and animations respectively
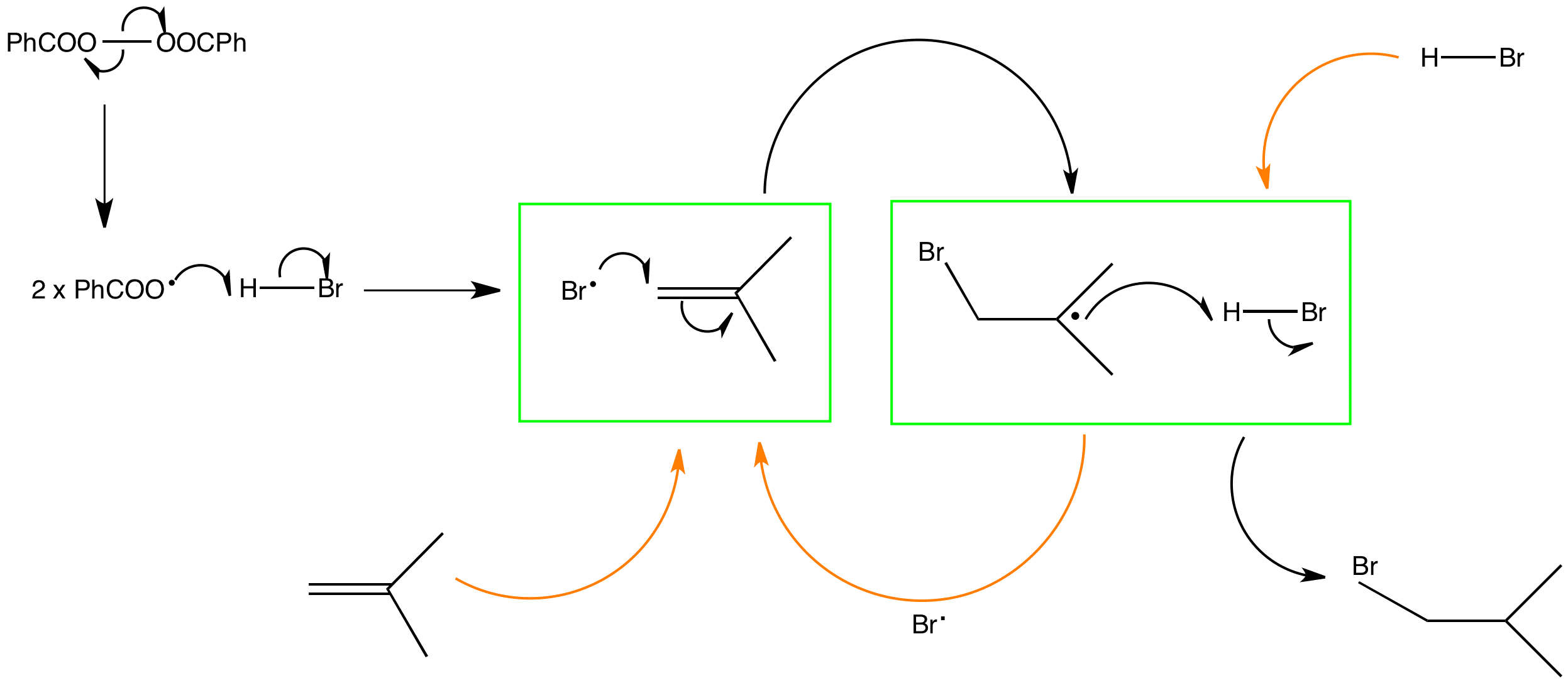
The chain is initiated using the homolysis of an O-O bond in dibenzoylperoxide. The benzoyl radicals can then abstract hydrogen from hydrogen bromide producing the key bromine radical in this reaction.
The propagation steps include the radical addition of bromine to the less hindered end of isobutene double bond to produce the more stabilised tertiary radical and the bromoisobutyl radical abstraction of hydrogen from hydrogen bromide propagating the chain.