Click the structures and reaction black arrows in sequence to view the 3D models and animations respectively
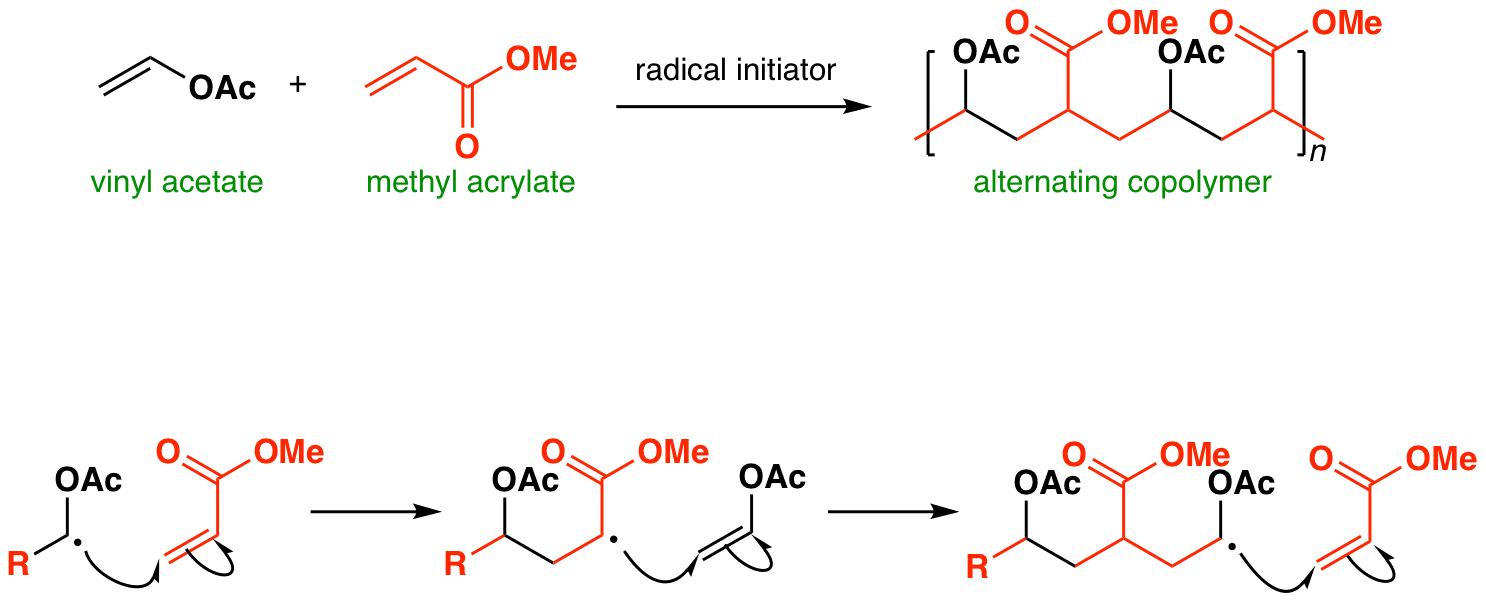
Radical chain reactions are particularly suited to the synthesis of polymers – this copolymerization demonstrates the effect of electron-withdrawing or donating substituents on radical reactivity.
When a mixture of vinyl acetate and methyl acrylate is treated with a radical initiator, polymerization takes place. The polymer produced contains alternating vinyl acetate and methyl acrylate monomers along its chain.
The animated mechanistic sequence above shows why – the nucleophilic radical from vinyl acetate (adjacent to filled n orbital of OAc; high energy SOMO) prefers to add to the electrophilic alkene (acrylate). The new radical (adjacent to the empty pi* orbital of CO2Me; low-energy SOMO) is electrophilic and prefers to add to the nucleophilic alkene (vinyl acetate). This produces a new nucleophilic radical, the cycle then occurs repeatedly until the desired chain length is produced.