Note:Click either the Acetone or Radical Anion and use the control buttons to view the HOMO, LUMO and Partial Atomic Charges
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
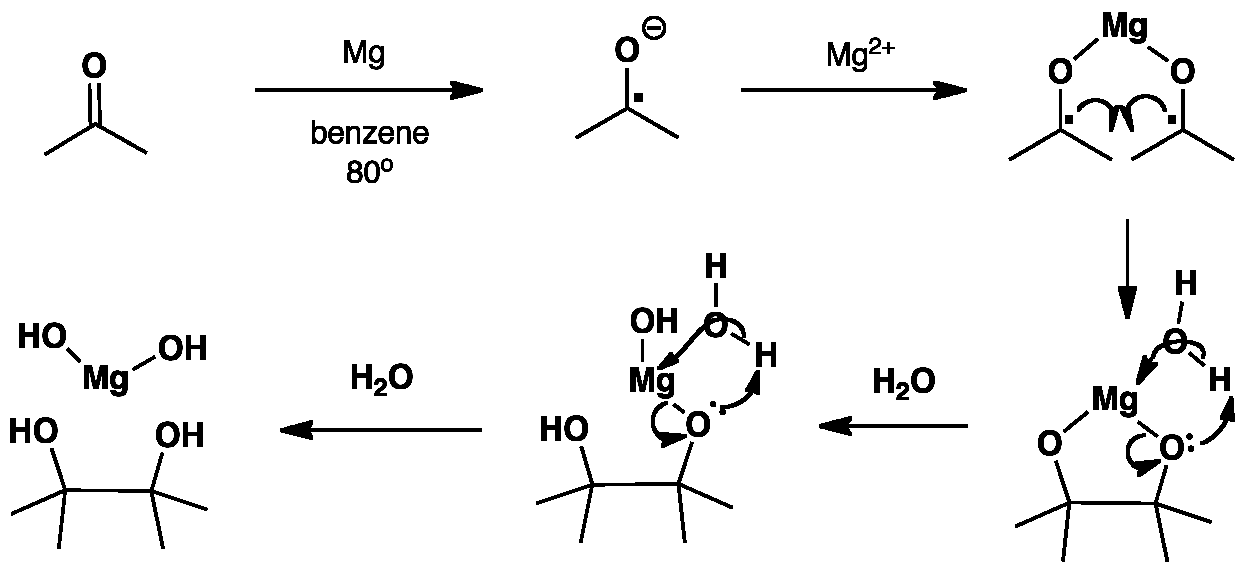
This example of a pinacol coupling shows the dimerisation of acetone to give a diol (2,3-dimethylbutane-2,3-diol). In aprotic solvents such as benzene the concentration of the ketyl radical builds up leading to dimerisation. The use of a metal such as magnesium or aluminium allows the reaction to occur more easily by forming covalent metal-oxygen bonds which reduces repulsion between anions.
Observe the difference in charge upon the oxygen in the ketyl radical compared to the acetone oxygen.
A. Chatterjee and N. N. Joshi, Tetrahedron, 2006, 62, 12137–12158.