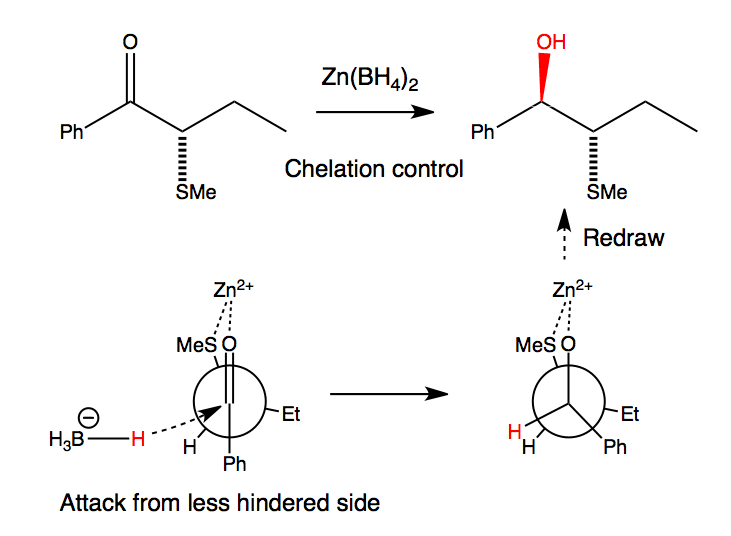
Zinc can chelate to the two heteroatoms with lone pairs, sulfur and the carbonyl group, and here it changes the conformation of the starting material. No longer does the most reactive or most populated conformation place the electronegative S atom perpendicular to the C=O; instead it prefers SR to lie as close to the carbonyl oxygen as possible so that Zn can bridge between S and O.
Attack of borohydride from the less hindered side (next to H rather than Et) leads to the anti diastereoisomer.
Link to non-chelated Felkin-Anh example