Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
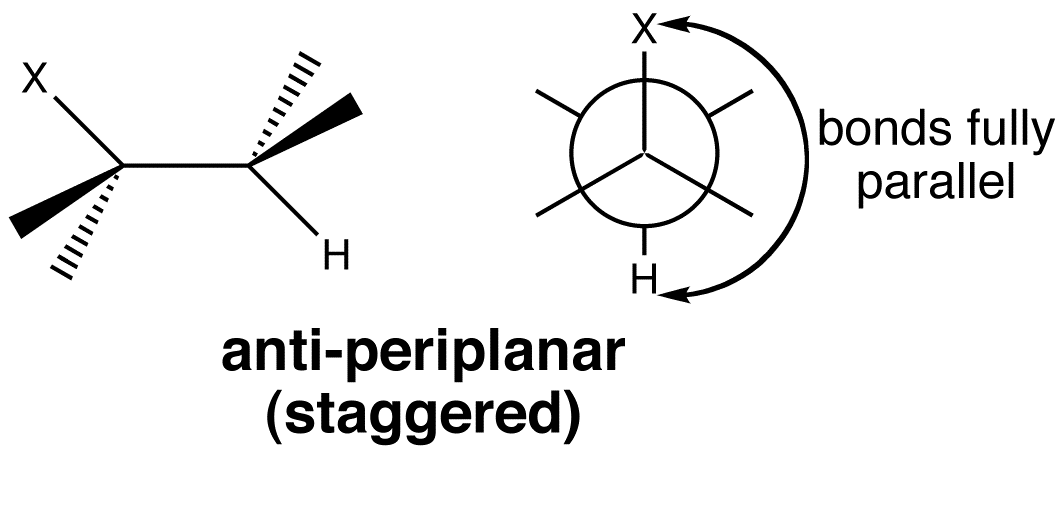
In an E2 eliminations, the new π bond is formed by overlap of the C-H σ bond with the C-X σ* anti-bonding orbital. The two orbitals have to lie in the same plane for best overlap. E2 elimination takes place from the anti-periplanar conformation, as this is the most stable conformation due to its staggered nature.
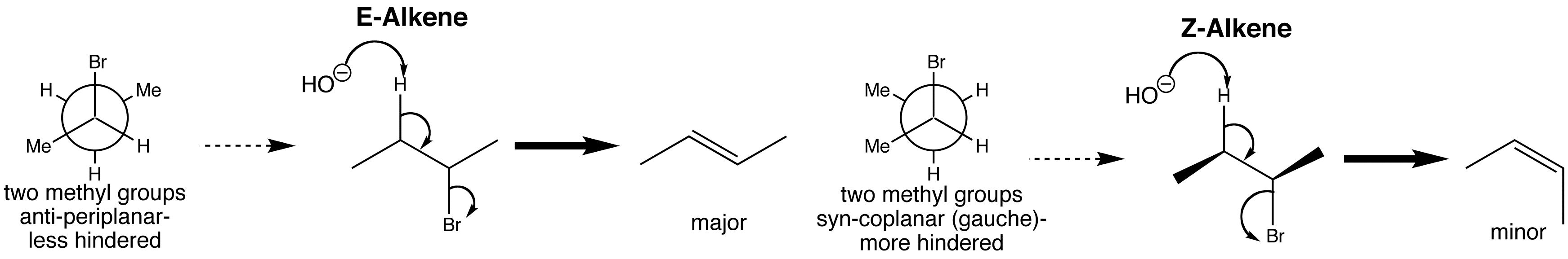
As you can see from the reaction below, when 2-bromobutane undergoes an E2 reaction, two possible stereoisomers are formed. This is because 2-bromobutane has two conformations with H and Br anti-periplanar, but the one that is less hindered forms the major product, so the E-alkene predominates.
The “Animated Molecular Orbital” is present to highlight the anti-periplanar nature of the leaving groups.
J. Sicher, Angew. Chemie Int. Ed. English, 1972, 11, 200–214.