Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
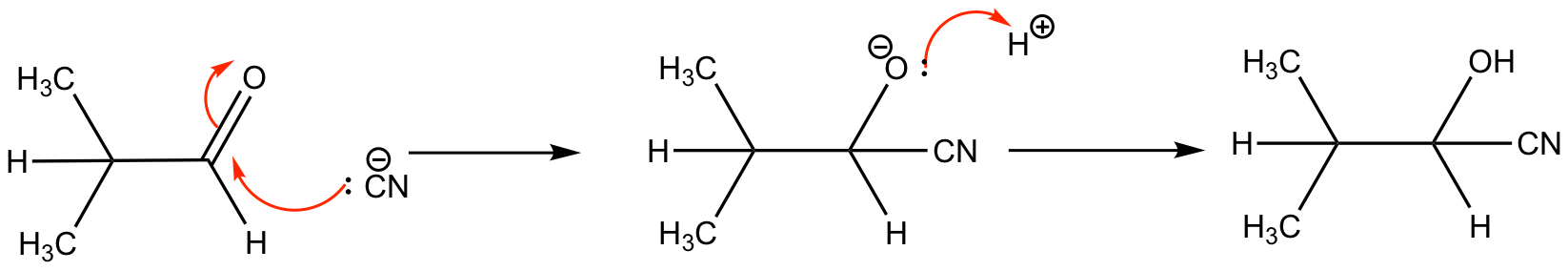
The cyanide functional group will add to a carbonyl as a nucleophile giving a hydroxynitrile (product). HCN is highly toxic, so the reactant is formed by adding dilute acid to sodium cyanide. This MUST be done in a fume cupboard. In the second step the oxyanion (intermediate) accepts a proton.
The product is optically inactive as cyanide can attack from either side (in equal probability).
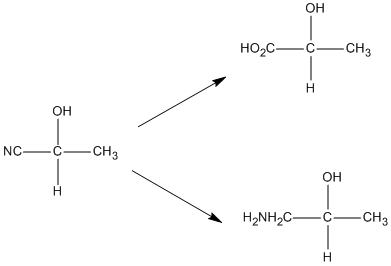
This reaction is synthetically useful as addition of acid can result in formation of a carboxylic acid. Catalytic hydrogenation reduced that cyanide group to an amine group. The reaction scheme for this is as follows: