NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
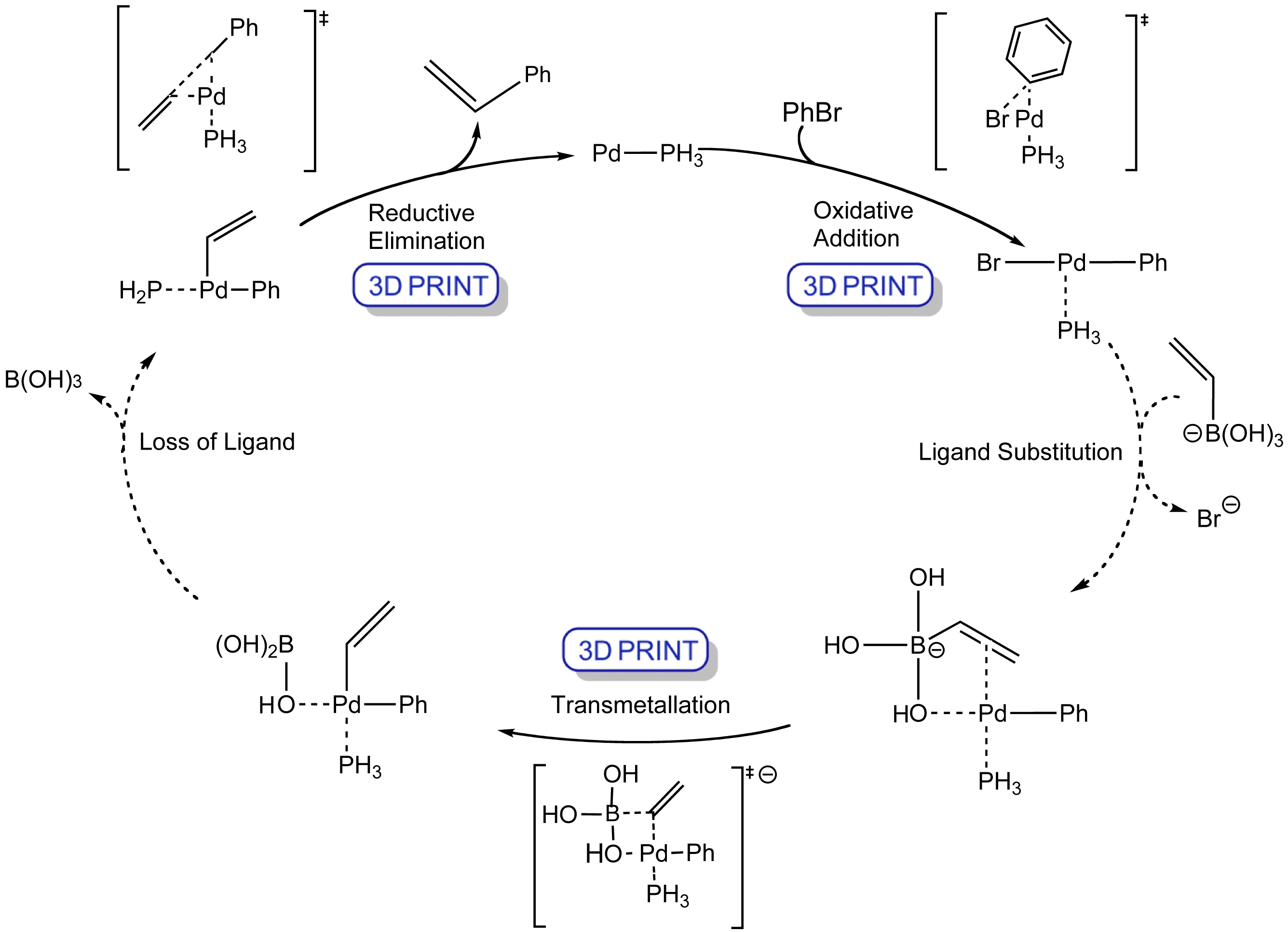
Click the structures and reaction arrows to view the 3D models and animations respectively
The Suzuki reaction is the palladium-catalysed addition of aryl, vinyl, or substituted vinyl groups to organic halides or triflates.
The mechanism involves the oxidative addition of the halide and a transmetallation with the boronate hydroxide and base which speeds up the reaction. The palladium(0) catalyst is then regenerated in the reductive elimination step to form the product.
A. Suzuki, J. Organomet. Chem., 1999, 576, 147–168.
F. Bellina, A. Carpita and R. Rossi, Synthesis (Stuttg)., 2004, 2004, 2419–2440.