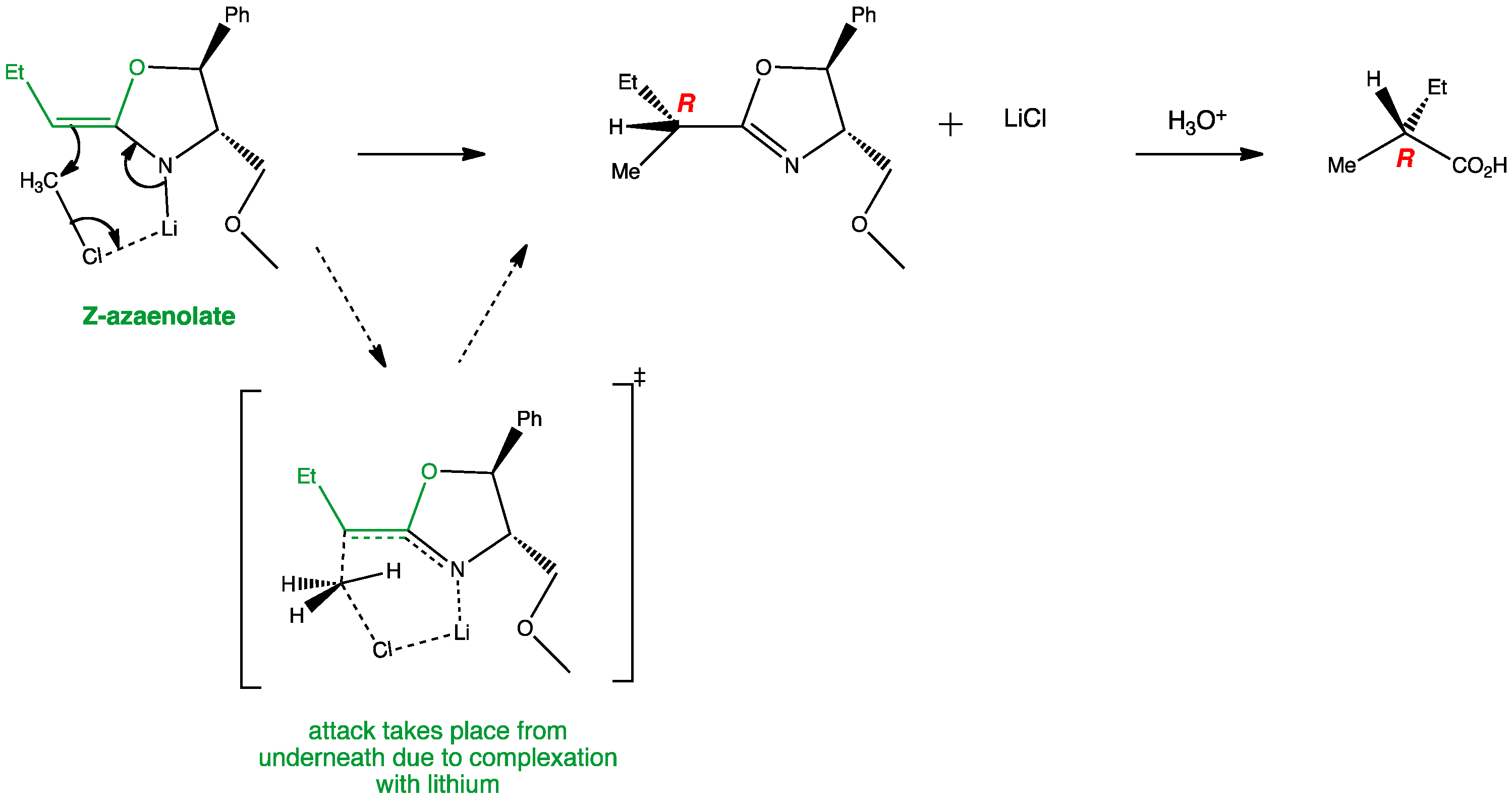
Carboxylic acids can be converted into chiral oxazolines which act as effective auxiliaries in diastereoselective alkylation of the enolate equivalents. The formation of Z-azaenolate along with the complexation of the electrophile, methyl chloride, with the lithium causes the attack to occur from underneath.The presence of a bulky group like phenyl also directs the electrophilic attack to take place at the bottom, therefore providing a single diastereoisomer of product and ultimately a single enantiomer of alkylated carboxylic acid.
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
A. I. Meyers and E. D. Mihelich, Angew. Chemie Int. Ed. English, 1976, 15, 270–281.