Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
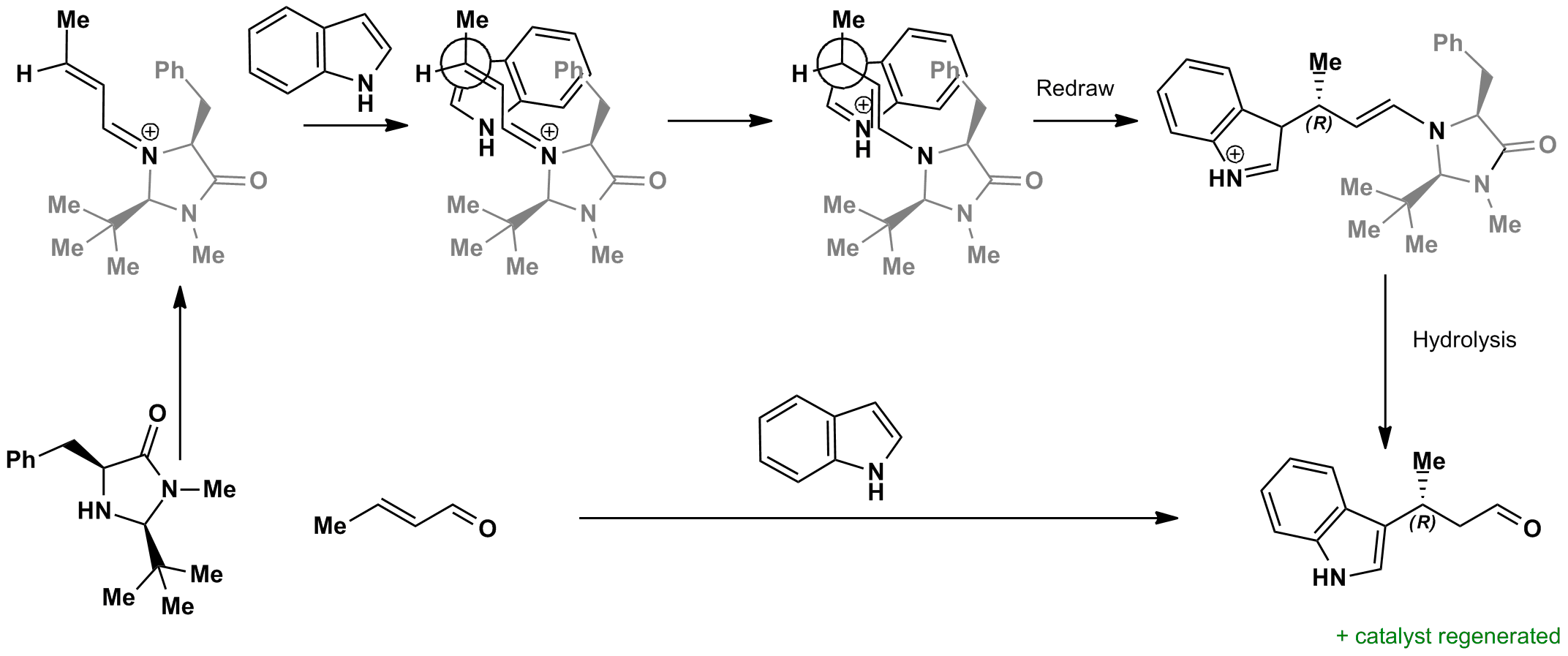
The chiral cataylst reacts with the carbonyl to form an imine. This shields one side of the molecule, forcing the indole to attack from one face only via a simple conjugate addition mechanism. The imine can then be hydrolysed, regenerating the catalyst and giving the carbonyl product.
J. F. Austin and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 1172–1173.