NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase.
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
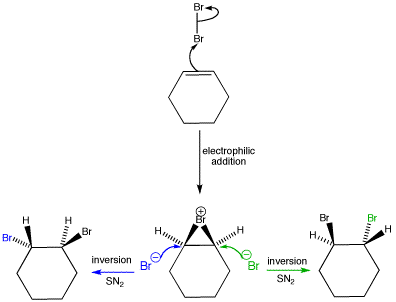
When cyclohexene is treated with bromine in carbon tetrachloride, the racemic anti-1,2-dibromocyclohexane is obtained exclusively. The bromonium ion is opened with inversion in an SN2 reaction. As the bromine can attack from either side we get both stereoisomers and a racemic mixture.