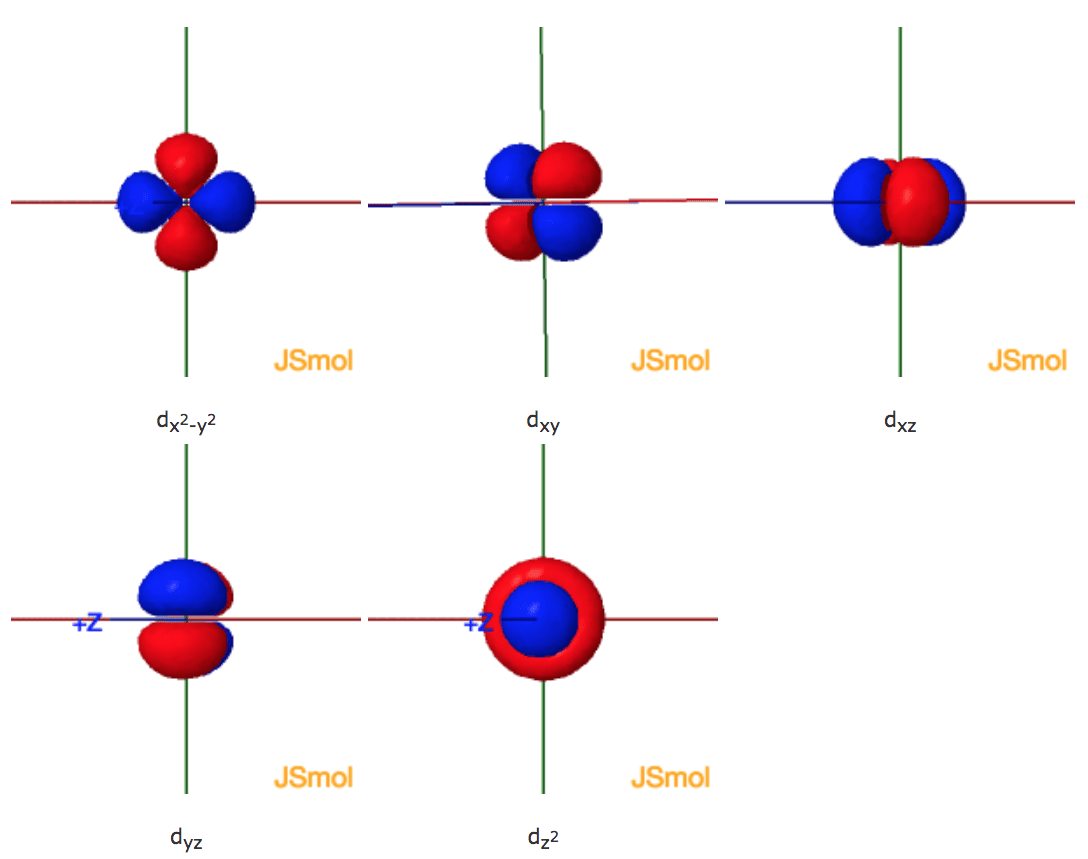
Click the images to see the various 3d orbitals. Nodal planes and one of the two nodal cones for dz2, where there is no electron density, are displayed after a short delay.
There are a total of five d orbitals and each orbital can hold two electrons. The transition metal series is defined by the progressive filling of the 3d orbitals.These five orbitals have the following ml values:
ml=0, ±1, ±2,
s-orbitals |2p-orbitals |3p-orbitals | 3d-orbitals | 4f-orbitals