Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
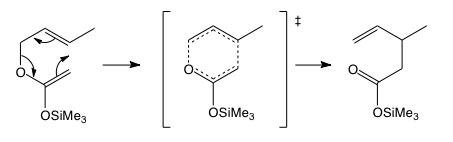
Why is the forward reaction favoured in the Claisen rearrangement if sigmatropic reactions are reversible? A carbonyl group results in the product (compare keto-enol stability).The [3,3] sigmatropic rearrangement can be further enhanced by sticking an oxygen outside the ring. This is employed in the Ireland-Claisen rearrangement, where a silyloxy group can be bolted on to the Claisen set up. This has wide applications synthetically, since the silyloxy group can be easily hydrolysed, with the achievement of a new C-C bond in the product.
Richard Windsor – Undergraduate Final Year Project 2008
Y. Chai, S. Hong, H. A. Lindsay, C. McFarland and M. C. McIntosh, Tetrahedron, 2002, 58, 2905–2928.