An enolate or enol equivalent can be added to an α,β-unsaturated carbonyl compound. This is an example of a simple base-catalysed enolization step:
Click the image to view the 3D animation
Enolate nucleophiles can undergo conjugate addition, but they have exactly the same opportunity to attack the carbonyl group directly as do simple nucleophiles. The same factors also govern the eventual outcome of the reaction. Thermodynamic control leads to conjugate addition, but kinetic control leads to direct attack. The key to successful conjugate addition is to ensure that direct addition to the carbonyl (which is an aldol reaction) is reversible. This enables the conjugate addition to compete and, as its product is more stable, it eventually becomes the sole product.
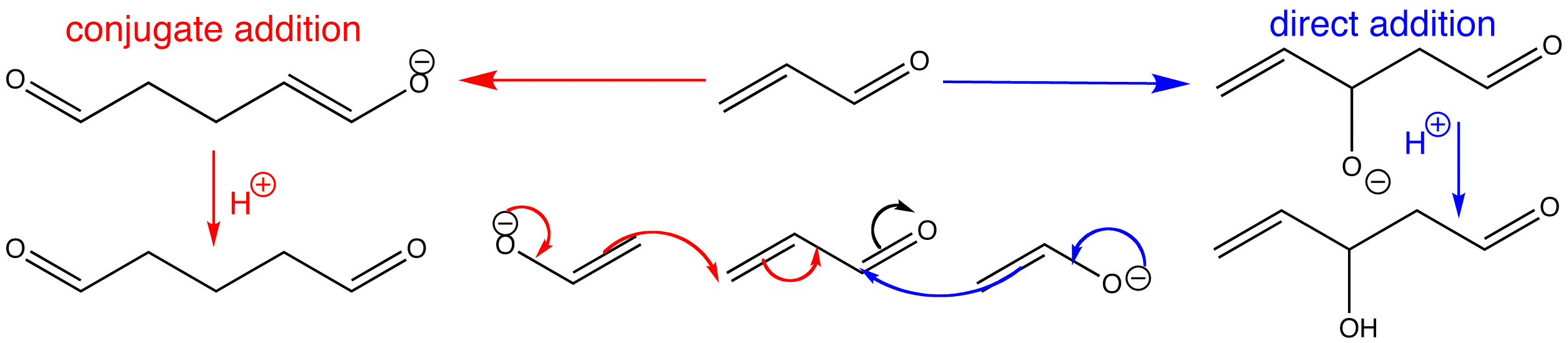
Click the two different types of reaction to view the 3D animations
A. G. Csákÿ, G. de la Herrán and M. C. Murcia, Chem. Soc. Rev., 2010, 39, 4080.