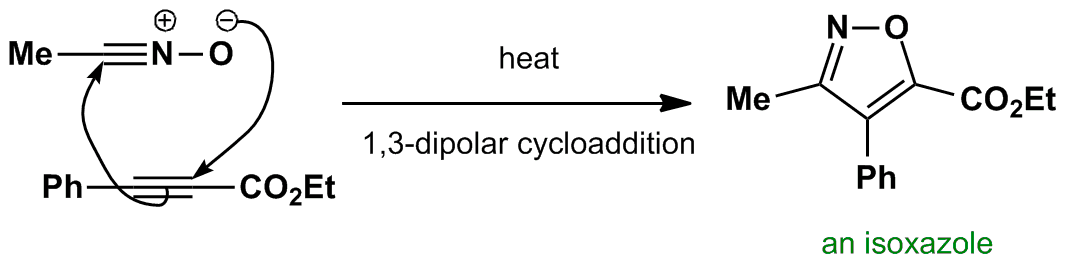
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The nitrile oxide is unstable so it is made in situ by reaction of hydroxylamine with an aldehyde. Click here to view this reaction. The nitrile oxide then reacts with the alkyne to produce the isoxazole.