ClF3 Chlorine Trifluoride
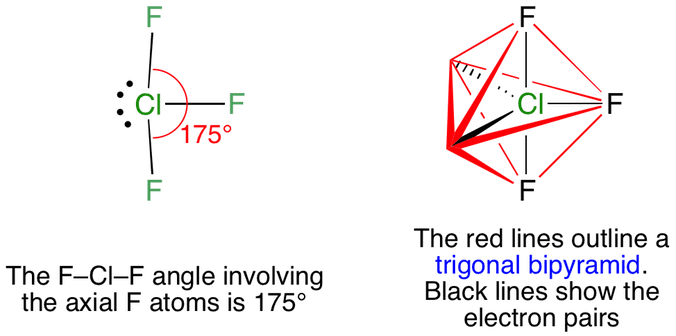
Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). These are arranged in a trigonal bipyramidal shape with a 175° F(axial)-Cl-F(axial) bond angle.
The two lone pairs take equatorial positions because they demand more space than the bonds. The result is a T-shaped molecule.
Click the structures to load the molecules
Related structures H2O | NH3 | CH4 | PF5 |SF4 |ClF3 | SF6 | XeF4




